Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach
- PMID: 33919084
- PMCID: PMC8143105
- DOI: 10.3390/plants10050825
Anti-Arthritic and Anti-Inflammatory Potential of Spondias mangifera Extract Fractions: An In Silico, In Vitro and In Vivo Approach
Abstract
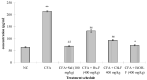
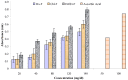
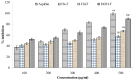
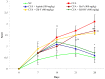
The fruits of Spondias mangifera (S. mangifera) have traditionally been used for the management of rheumatism in the northeast region of India. The present study explores the probable anti-arthritis and anti-inflammatory potential of S. mangifera fruit extract's ethanolic fraction (EtoH-F). To support this study, we first approached the parameters in silico by means of the active constituents of the plant (beta amyrin, beta sitosterol, oleonolic acid and co-crystallised ligands, i.e., SPD-304) via molecular docking on COX-1, COX-2 and TNF-α. Thereafter, the absorption, distribution, metabolism, excretion and toxicity properties were also determined, and finally experimental activity was performed in vitro and in vivo. The in vitro activities of the plant extract fractions were evaluated by means of parameters like 1,1-Diphenyl-2- picrylhydrazyl (DPPH), free radical-reducing potential, albumin denaturation, and protease inhibitory activity. The in vivo activity was evaluated using parameters like COX, TNF-α and IL-6 inhibition assay and arthritis score in Freund Adjuvant (CFA) models at a dose of 400 mg/kg b.w. per day of different fractions (hexane, chloroform, alcoholic). The molecular docking assay was performed on COX-1, COX-2 and TNF-α. The results of in vitro studies showed concentration-dependent reduction in albumin denaturation, protease inhibitors and scavenging activity at 500 µg/mL. Administration of the S. mangifera alcoholic fraction at the abovementioned dose resulted in a significant reduction (p < 0.01) in arthritis score, paw diameters, TNF-α, IL-6 as compared to diseased animals. The docking results showed that residues show a critical binding affinity with TNF-α and act as the TNF-α antagonist. The alcoholic fraction of S. mangifera extract possesses beneficial effects on rheumatoid arthritis as well as anti-inflammatory potential, and can further can be used as a possible agent for novel target-based therapies for the management of arthritis.
Keywords: Spondias mangifera; anti-inflammatory; arthritis; in silico; in vitro and in vivo.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Sowjanya R., Shankar M., Sireesha B., Naik E.A., Yudharaj P., Priyadarshini R.J. An overview on inflammation and plant having anti-inflammatory activity. Int. J. Phytopharm. Res. 2017;7:25–32.
-
- Marquardt P., Seide R., Vissiennon C., Schubert A., Birkemeyer C., Ahyi V., Fester K. Phytochemical characterization and in-vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules. 2020;25:288. doi: 10.3390/molecules25020288. - DOI - PMC - PubMed
-
- Zhang Q., Yu Y., Li J., Guan Y., Huang J., Wang Z., Zhang Z., Zhang W., Guo J., Li J., et al. Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc. (whole plant) in rodents. J. Ethnopharmacol. 2018;225:359–366. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

