Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review
- PMID: 33919147
- PMCID: PMC8122410
- DOI: 10.3390/ijms22094306
Role and Modulation of TRPV1 in Mammalian Spermatozoa: An Updated Review
Abstract
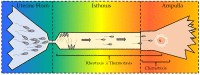
Based on the abundance of scientific publications, the polymodal sensor TRPV1 is known as one of the most studied proteins within the TRP channel family. This receptor has been found in numerous cell types from different species as well as in spermatozoa. The present review is focused on analyzing the role played by this important channel in the post-ejaculatory life of spermatozoa, where it has been described to be involved in events such as capacitation, acrosome reaction, calcium trafficking, sperm migration, and fertilization. By performing an exhaustive bibliographic search, this review gathers, for the first time, all the modulators of the TRPV1 function that, to our knowledge, were described to date in different species and cell types. Moreover, all those modulators with a relationship with the reproductive process, either found in the female tract, seminal plasma, or spermatozoa, are presented here. Since the sperm migration through the female reproductive tract is one of the most intriguing and less understood events of the fertilization process, in the present work, chemotaxis, thermotaxis, and rheotaxis guiding mechanisms and their relationship with TRPV1 receptor are deeply analyzed, hypothesizing its (in)direct participation during the sperm migration. Last, TRPV1 is presented as a pharmacological target, with a special focus on humans and some pathologies in mammals strictly related to the male reproductive system.
Keywords: TRPV1; chemotaxis; endocannabinoid system; human; ion channel; mammals; sperm signaling; spermatozoa; thermotaxis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

