Nusinersen Modulates Proteomics Profiles of Cerebrospinal Fluid in Spinal Muscular Atrophy Type 1 Patients
- PMID: 33919289
- PMCID: PMC8122268
- DOI: 10.3390/ijms22094329
Nusinersen Modulates Proteomics Profiles of Cerebrospinal Fluid in Spinal Muscular Atrophy Type 1 Patients
Abstract
Spinal muscular atrophy (SMA) type 1 is a severe infantile autosomal-recessive neuromuscular disorder caused by a survival motor neuron 1 gene (SMN1) mutation and characterized by progressive muscle weakness. Without supportive care, SMA type 1 is rapidly fatal. The antisense oligonucleotide nusinersen has recently improved the natural course of this disease. Here, we investigated, with a functional proteomic approach, cerebrospinal fluid (CSF) protein profiles from SMA type 1 patients who underwent nusinersen administration to clarify the biochemical response to the treatment and to monitor disease progression based on therapy. Six months after starting treatment (12 mg/5 mL × four doses of loading regimen administered at days 0, 14, 28, and 63), we observed a generalized reversion trend of the CSF protein pattern from our patient cohort to that of control donors. Notably, a marked up-regulation of apolipoprotein A1 and apolipoprotein E and a consistent variation in transthyretin proteoform occurrence were detected. Since these multifunctional proteins are critically active in biomolecular processes aberrant in SMA, i.e., synaptogenesis and neurite growth, neuronal survival and plasticity, inflammation, and oxidative stress control, their nusinersen induced modulation may support SMN improved-expression effects. Hence, these lipoproteins and transthyretin could represent valuable biomarkers to assess patient responsiveness and disease progression.
Keywords: ASO; apolipoprotein A1; apolipoprotein E; carbonyl groups; haptoglobin; neuromuscular disease; nusinersen; oxidized proteins; spinal muscular atrophy type 1; survival motor neuron (SMN); transthyretin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

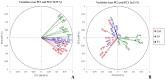
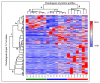
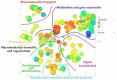



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

