Synthesis of New Triazolopyrazine Antimalarial Compounds
- PMID: 33919319
- PMCID: PMC8122397
- DOI: 10.3390/molecules26092421
Synthesis of New Triazolopyrazine Antimalarial Compounds
Abstract
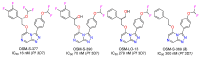
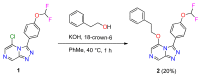
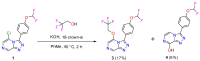
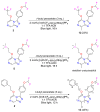
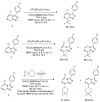
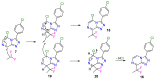
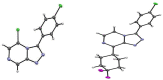
A radical approach to late-stage functionalization using photoredox and Diversinate™ chemistry on the Open Source Malaria (OSM) triazolopyrazine scaffold (Series 4) resulted in the synthesis of 12 new analogues, which were characterized by NMR, UV, and MS data analysis. The structures of four triazolopyrazines were confirmed by X-ray crystal structure analysis. Several minor and unexpected side products were generated during these studies, including two resulting from a possible disproportionation reaction. All compounds were tested for their ability to inhibit the growth of the malaria parasite Plasmodium falciparum (3D7 and Dd2 strains) and for cytotoxicity against a human embryonic kidney (HEK293) cell line. Moderate antimalarial activity was observed for some of the compounds, with IC50 values ranging from 0.3 to >20 µM; none of the compounds displayed any toxicity against HEK293 at 80 µM.
Keywords: Diversinate™; Open Source Malaria; X-ray; antimalarial; difluoroethylation; drug discovery; late-stage functionalization; mechanism; methylation; photoredox; radical disproportionation; synthesis; triazolopyrazine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Shapiro G. 3,3-Difluoropiperidine Carbamate Heterocyclic Compounds as NR2B NMDA Receptor Antagonists. 10294230B2. U.S. Patent. 2015 Jun 1;
-
- Open Source Malaria Project wiki; Open Source Malaria project/Series 4. [(accessed on 20 January 2021)]; Available online: https://github.com/OpenSourceMalaria/Series4/wiki.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

