Identification of Tumor-Specific MRI Biomarkers Using Machine Learning (ML)
- PMID: 33919342
- PMCID: PMC8143297
- DOI: 10.3390/diagnostics11050742
Identification of Tumor-Specific MRI Biomarkers Using Machine Learning (ML)
Abstract
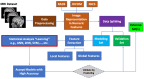
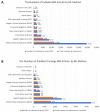
The identification of reliable and non-invasive oncology biomarkers remains a main priority in healthcare. There are only a few biomarkers that have been approved as diagnostic for cancer. The most frequently used cancer biomarkers are derived from either biological materials or imaging data. Most cancer biomarkers suffer from a lack of high specificity. However, the latest advancements in machine learning (ML) and artificial intelligence (AI) have enabled the identification of highly predictive, disease-specific biomarkers. Such biomarkers can be used to diagnose cancer patients, to predict cancer prognosis, or even to predict treatment efficacy. Herein, we provide a summary of the current status of developing and applying Magnetic resonance imaging (MRI) biomarkers in cancer care. We focus on all aspects of MRI biomarkers, starting from MRI data collection, preprocessing and machine learning methods, and ending with summarizing the types of existing biomarkers and their clinical applications in different cancer types.
Keywords: MRI; biomarkers; imaging; machine learning; oncology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Boellaard R., Delgado-Bolton R., Oyen W.J.G., Giammarile F., Tatsch K., Eschner W., Verzijlbergen F.J., Barrington S.F., Pike L.C., Weber W.A., et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. - DOI - PMC - PubMed
-
- Barentsz J.O., Weinreb J.C., Verma S., Thoeny H.C., Tempany C.M., Shtern F., Padhani A.R., Margolis D., Macura K.J., Haider M.A., et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur. Urol. 2016;69:41–49. doi: 10.1016/j.eururo.2015.08.038. - DOI - PMC - PubMed
-
- DeSouza N.M., Achten E., Alberich-Bayarri A., Bamberg F., Boellaard R., Clément O., Fournier L., Gallagher F., Golay X., Heussel C.P., et al. Validated Imaging Biomarkers as Decision-Making Tools in Clinical Trials and Routine Practice: Current Status and Recommendations from the EIBALL* Subcommittee of the European Society of Radiology (ESR) Insights Imaging. 2019;10:1–6. doi: 10.1186/s13244-019-0764-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

