Obesity Drives an Oral Microbiota Signature of Female Patients with Periodontitis: A Pilot Study
- PMID: 33919425
- PMCID: PMC8143370
- DOI: 10.3390/diagnostics11050745
Obesity Drives an Oral Microbiota Signature of Female Patients with Periodontitis: A Pilot Study
Abstract
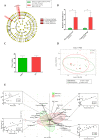
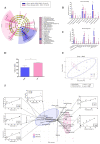
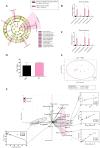
The aim of this study was to analyze the link between oral microbiota and obesity in humans. We conducted a pilot study including 19 subjects with periodontitis divided into two groups: normo-weighted subjects (NWS) with a body mass index (BMI) between 20 and 25 (n = 9) and obese subjects (OS) with a BMI > 30 (n = 10). Obesity was associated with a poor oral health status characterized by an increased number of missing teeth and a higher score of periodontal-support loss associated with dysbiotic oral microbiota (39.45 ± 3.74 vs. 26.41 ± 11.21, p = 0.03 for the Chao 1 index). Oral microbiota taxonomic analysis showed that the abundance of the Capnocytophaga genus was higher (2.47% ± 3.02 vs. 0.27% ± 0.29, p = 0.04) in OS compared to NWS. Obese females (OF) were characterized by an increase in the Streptococcus genus (34.12% ± 14.29 vs. 10.55% ± 10.42, p = 0.05) compared to obese males (OM), where the Neisseria genus was increased (5.75% ± 5.03 vs. 58.05% ± 30.64, p = 0.008). These first data suggest that sex/gender is determinant in the link between oral dysbiotic microbiota and obesity in patients with periodontitis. Our results could lead to recommendations concerning therapeutic strategies for obese patients with periodontitis following the sex/gender.
Keywords: dysbiosis; obesity; oral microbiota; periodontitis; sex/gender.
Conflict of interest statement
The authors declare that they have no competing interest and no conflict of interests.
Figures

References
-
- Eschwege E., Charles M., Basdevant A. ObEpi Enquête Epidémiologique Nationale Sur Le Surpoid et l’obésité. Inserm, Kanthar Healthe, Roche. [(accessed on 10 May 2020)];2012 Available online: https://www.roche.fr/content/dam/rochexx/roche-fr/roche_france/fr_FR/doc....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

