HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models
- PMID: 33919468
- PMCID: PMC8122524
- DOI: 10.3390/cancers13092007
HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models
Abstract
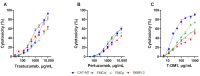
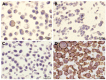
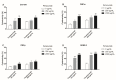
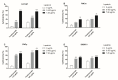
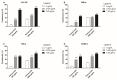
Feline mammary carcinoma (FMC) is a highly prevalent tumor, showing aggressive clinicopathological features, with HER2-positive being the most frequent subtype. While, in human breast cancer, the use of anti-HER2 monoclonal antibodies (mAbs) is common, acting by blocking the extracellular domain (ECD) of the HER2 protein and by inducing cell apoptosis, scarce information is available on use these immunoagents in FMC. Thus, the antiproliferative effects of two mAbs (trastuzumab and pertuzumab), of an antibody-drug conjugate compound (T-DM1) and of combined treatments with a tyrosine kinase inhibitor (lapatinib) were evaluated on three FMC cell lines (CAT-MT, FMCm and FMCp). In parallel, the DNA sequence of the her2 ECD (subdomains II and IV) was analyzed in 40 clinical samples of FMC, in order to identify mutations, which can lead to antibody resistance or be used as prognostic biomarkers. Results obtained revealed a strong antiproliferative effect in all feline cell lines, and a synergistic response was observed when combined therapies were performed. Additionally, the mutations found were not described as inducing resistance to therapy in breast cancer patients. Altogether, our results suggested that anti-HER2 mAbs could become useful in the treatment of FMC, particularly, if combined with lapatinib, since drug-resistance seems to be rare.
Keywords: HER2; combined therapies; feline her2 mutations; feline mammary carcinoma; monoclonal antibodies; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Tyrosine Kinase Inhibitors Are Promising Therapeutic Tools for Cats with HER2-Positive Mammary Carcinoma.Pharmaceutics. 2021 Mar 6;13(3):346. doi: 10.3390/pharmaceutics13030346. Pharmaceutics. 2021. PMID: 33800900 Free PMC article.
-
HER-targeted tyrosine kinase inhibitors enhance response to trastuzumab and pertuzumab in HER2-positive breast cancer.Invest New Drugs. 2019 Jun;37(3):441-451. doi: 10.1007/s10637-018-0649-y. Epub 2018 Jul 30. Invest New Drugs. 2019. PMID: 30062574
-
Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer.Cancer Res. 2005 Feb 1;65(3):907-12. Cancer Res. 2005. PMID: 15705889
-
Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates.Ann Transl Med. 2014 Dec;2(12):122. doi: 10.3978/j.issn.2305-5839.2014.08.13. Ann Transl Med. 2014. PMID: 25568875 Free PMC article. Review.
-
Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma.Vet Sci. 2021 Aug 11;8(8):164. doi: 10.3390/vetsci8080164. Vet Sci. 2021. PMID: 34437486 Free PMC article. Review.
Cited by
-
TIM-3 Is a Potential Immune Checkpoint Target in Cats with Mammary Carcinoma.Cancers (Basel). 2023 Jan 6;15(2):384. doi: 10.3390/cancers15020384. Cancers (Basel). 2023. PMID: 36672332 Free PMC article.
-
Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future.Vet Sci. 2024 May 2;11(5):199. doi: 10.3390/vetsci11050199. Vet Sci. 2024. PMID: 38787171 Free PMC article. Review.
-
Recent Issues in the Development and Application of Targeted Therapies with Respect to Individual Animal Variability.Animals (Basel). 2025 Feb 6;15(3):444. doi: 10.3390/ani15030444. Animals (Basel). 2025. PMID: 39943214 Free PMC article. Review.
-
Effects of Lapatinib on HER2-Positive and HER2-Negative Canine Mammary Carcinoma Cells Cultured In Vitro.Pharmaceutics. 2021 Jun 17;13(6):897. doi: 10.3390/pharmaceutics13060897. Pharmaceutics. 2021. PMID: 34204236 Free PMC article.
-
Purification and In Vitro Evaluation of an Anti-HER2 Affibody-Monomethyl Auristatin E Conjugate in HER2-Positive Cancer Cells.Biology (Basel). 2021 Aug 7;10(8):758. doi: 10.3390/biology10080758. Biology (Basel). 2021. PMID: 34439990 Free PMC article.
References
-
- Soares M., Correia J., Rodrigues P., Simões M., Matos A., de Ferreira F. Feline HER2 Protein Expression Levels and Gene Status in Feline Mammary Carcinoma: Optimization of Immunohistochemistry (IHC) and In Situ Hybridization (ISH) Techniques. Microsc. Microanal. 2013;19:876–882. doi: 10.1017/S1431927613001529. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

