Development of a Method for Simultaneous Analysis of Allergenic Flavoring Agents in Cigarettes and Quantitative Risk Assessment for Consumer Safety
- PMID: 33919504
- PMCID: PMC8072964
- DOI: 10.3390/toxics9040087
Development of a Method for Simultaneous Analysis of Allergenic Flavoring Agents in Cigarettes and Quantitative Risk Assessment for Consumer Safety
Abstract
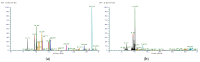
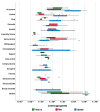
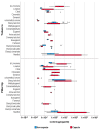
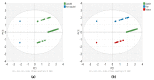
Flavoring agents are added to cigarettes to improve taste. There are mostly permitted food additives, but some of them are restricted for use in food, cosmetics, and toys, since they can cause allergic reactions. Previous studies have investigated the levels of flavoring agents in tobacco but none has focused on their content in filter tips and capsules. Moreover, no studies have assessed the risk of adding allergenic flavoring agents in cigarettes. Here, we developed and validated a simultaneous analysis method for 25 allergenic flavoring agents and menthol with gas chromatography-tandem mass spectrometry to determine levels of flavoring agents in the tobacco, filter tips, and capsules of 54 commercial cigarettes in Korea. All cigarettes contained at least one allergenic flavoring agent regardless of the inclusion of flavoring capsules. Importantly, the filter tips and the capsules contained higher levels of flavoring agents than tobacco, highlighting the importance of the quantification of flavoring agents in these parts of cigarettes. Nevertheless, the risk assessment based on their levels in cigarettes suggested that their exposure was maintained at a safe level. However, the risk assessed from maximum menthol, linalool, and cinnamaldehyde exceeded one-tenth of derived no-effect levels, suggesting the need for further studies on their risk to human health.
Keywords: allergenic flavoring agent; capsule cigarette; gas chromatography-tandem mass spectrometry; inhalation exposure; risk assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Quantitative analysis of menthol and identification of other flavoring ingredients in capsule cigarettes marketed in Korea.Regul Toxicol Pharmacol. 2018 Feb;92:420-428. doi: 10.1016/j.yrtph.2018.01.002. Epub 2018 Jan 6. Regul Toxicol Pharmacol. 2018. PMID: 29309808
-
Chemicals in Cigarette Flavor Capsules From Guatemala and Mexico.Nicotine Tob Res. 2024 Apr 22;26(5):545-551. doi: 10.1093/ntr/ntad216. Nicotine Tob Res. 2024. PMID: 37930843 Free PMC article.
-
Effect of cigarette menthol content on mainstream smoke emissions.Chem Res Toxicol. 2011 Oct 17;24(10):1744-53. doi: 10.1021/tx200285s. Epub 2011 Sep 19. Chem Res Toxicol. 2011. PMID: 21888394
-
Menthol additives to tobacco products. Reasons for withdrawing mentholated cigarettes in European Union on 20th may 2020 according to tobacco products directive (2014/40/EU).Toxicol Mech Methods. 2020 Oct;30(8):555-561. doi: 10.1080/15376516.2020.1805662. Epub 2020 Aug 14. Toxicol Mech Methods. 2020. PMID: 32746758 Review.
-
Impact of non-menthol flavours in e-cigarettes on perceptions and use: an updated systematic review.BMJ Open. 2019 Oct 16;9(10):e031598. doi: 10.1136/bmjopen-2019-031598. BMJ Open. 2019. PMID: 31619431 Free PMC article.
References
-
- FDA (Food and Drug Administration) Substances Added to Food (Formerly EAFUS) [(accessed on 30 October 2020)]; Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FoodSubstances.
-
- FEMA Expert Panel Flavor Ingredient Library. [(accessed on 20 February 2020)]; Available online: https://www.femaflavor.org/flavor-library.
-
- Nair U. Additives in Tobacco Products: Contribution of Carob Bean Extract, Cellulose Fibre, Guar Gum, Liquorice, Menthol, Prune Juice Concentrate and Vanillin to Attractiveness, Addictiveness and Toxicity of Tobacco Smoking. German Cancer Research Center; Heidelberg, Germany: 2012. pp. 1–31.
-
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) Final Opinion on the Additives Used in Tobacco Products. SCENIHR; Luxembourg, Luxembourg: 2016. - DOI
-
- European Commission Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L. 2009;342:59–208.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

