The New Serum-Free OptiPASS® Medium in Cold and Oxygen-Free Conditions: An Innovative Conservation Method for the Preservation of MDA-MB-231 Triple Negative Breast Cancer Spheroids
- PMID: 33919619
- PMCID: PMC8073891
- DOI: 10.3390/cancers13081945
The New Serum-Free OptiPASS® Medium in Cold and Oxygen-Free Conditions: An Innovative Conservation Method for the Preservation of MDA-MB-231 Triple Negative Breast Cancer Spheroids
Abstract
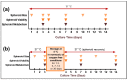
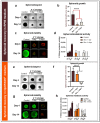
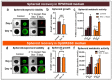
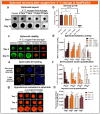
Cancer spheroids are very effective preclinical models to improve anticancer drug screening. In order to optimize and extend the use of spheroid models, these works were focused on the development of a new storage concept to maintain these models in the longer term using the Triple-Negative Breast Cancer MDA-MB-231 spheroid models. The results highlight that the combination of a temperature of 4 °C and oxygen-free conditions allowed the spheroid characteristics of OptiPASS® serum-free culture medium to preserve the spheroid characteristics during 3-, 5- or 7-day-long storage. Indeed, after storage they were returned to normal culture conditions, with recovered spheroids presenting similar growth rates (recovery = 96.2%), viability (Live/Dead® profiles) and metabolic activities (recovery = 90.4%) compared to nonstored control spheroids. Likewise, both recovered spheroids (after storage) and nonstored controls presented the same response profiles as two conventional drugs, i.e., epirubicin and cisplatin, and two anti-PARP1 targeted drugs-i.e., olaparib and veliparib. This new original storage concept seems to induce a temporary stop in spheroid growth while maintaining their principal characteristics for further use. In this way, this innovative and simple storage concept may instigate future biological sample preservation strategies.
Keywords: MDA-MB-231 spheroids; drug screening; new spheroid storage concept; preclinical spheroid models; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The New Synthetic Serum-Free Medium OptiPASS Promotes High Proliferation and Drug Efficacy Prediction on Spheroids from MDA-MB-231 and SUM1315 Triple-Negative Breast Cancer Cell Lines.J Clin Med. 2019 Mar 21;8(3):397. doi: 10.3390/jcm8030397. J Clin Med. 2019. PMID: 30901969 Free PMC article.
-
Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model.Onco Targets Ther. 2020 Jun 11;13:5395-5405. doi: 10.2147/OTT.S249756. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606757 Free PMC article.
-
Cold storage of porcine hepatocyte spheroids for spheroid bioartificial liver.Xenotransplantation. 2019 Jul;26(4):e12512. doi: 10.1111/xen.12512. Epub 2019 Apr 10. Xenotransplantation. 2019. PMID: 30968460 Free PMC article.
-
Bone Sialoprotein Shows Enhanced Expression in Early, High-Proliferation Stages of Three-Dimensional Spheroid Cell Cultures of Breast Cancer Cell Line MDA-MB-231.Front Oncol. 2019 Feb 5;9:36. doi: 10.3389/fonc.2019.00036. eCollection 2019. Front Oncol. 2019. PMID: 30805306 Free PMC article.
-
Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols.J Mammary Gland Biol Neoplasia. 2016 Dec;21(3-4):89-98. doi: 10.1007/s10911-016-9359-2. Epub 2016 Aug 12. J Mammary Gland Biol Neoplasia. 2016. PMID: 27518775 Review.
Cited by
-
LightSpot®-FL-1 Fluorescent Probe: An Innovative Tool for Cancer Drug Resistance Analysis by Direct Detection and Quantification of the P-glycoprotein (P-gp) on Monolayer Culture and Spheroid Triple Negative Breast Cancer Models.Cancers (Basel). 2021 Aug 11;13(16):4050. doi: 10.3390/cancers13164050. Cancers (Basel). 2021. PMID: 34439204 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

