Conquering the Nuclear Envelope Barriers by EBV Lytic Replication
- PMID: 33919628
- PMCID: PMC8073350
- DOI: 10.3390/v13040702
Conquering the Nuclear Envelope Barriers by EBV Lytic Replication
Erratum in
-
Correction: Lee, C.-P.; Chen, M.-R. Conquering the Nuclear Envelope Barriers by EBV Lytic Replication. Viruses 2021, 13, 702.Viruses. 2023 Sep 25;15(10):1991. doi: 10.3390/v15101991. Viruses. 2023. PMID: 37896914 Free PMC article.
Abstract
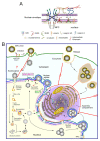
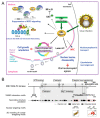
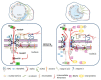
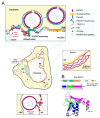
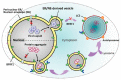
The nuclear envelope (NE) of eukaryotic cells has a highly structural architecture, comprising double lipid-bilayer membranes, nuclear pore complexes, and an underlying nuclear lamina network. The NE structure is held in place through the membrane-bound LINC (linker of nucleoskeleton and cytoskeleton) complex, spanning the inner and outer nuclear membranes. The NE functions as a barrier between the nucleus and cytoplasm and as a transverse scaffold for various cellular processes. Epstein-Barr virus (EBV) is a human pathogen that infects most of the world's population and is associated with several well-known malignancies. Within the nucleus, the replicated viral DNA is packaged into capsids, which subsequently egress from the nucleus into the cytoplasm for tegumentation and final envelopment. There is increasing evidence that viral lytic gene expression or replication contributes to the pathogenesis of EBV. Various EBV lytic proteins regulate and modulate the nuclear envelope structure in different ways, especially the viral BGLF4 kinase and the nuclear egress complex BFRF1/BFRF2. From the aspects of nuclear membrane structure, viral components, and fundamental nucleocytoplasmic transport controls, this review summarizes our findings and recently updated information on NE structure modification and NE-related cellular processes mediated by EBV.
Keywords: BFRF1; BGLF4 kinase; Epstein–Barr virus; nuclear egress; nuclear envelope modulation.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
-
- Moir R.D., Spann T.P., Goldman R.D. The dynamic properties and possible functions of nuclear lamins. Int. Rev. Cytol. 1995;162B:141–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

