The Use of Dual Cyclodextrin Chiral Selector Systems in the Enantioseparation of Pharmaceuticals by Capillary Electrophoresis: An Overview
- PMID: 33919692
- PMCID: PMC8069766
- DOI: 10.3390/molecules26082261
The Use of Dual Cyclodextrin Chiral Selector Systems in the Enantioseparation of Pharmaceuticals by Capillary Electrophoresis: An Overview
Abstract
Cyclodextrin (CD) derivatives are the most efficient and frequently used chiral selectors (CSs) in capillary electrophoresis (CE). There are situations when the use of a single CD as CS is not enough to obtain efficient chiral discrimination of the enantiomers; in these cases, sometimes this problem can be resolved using a dual CD system. The use of dual CD systems can often dramatically enhance enantioseparation selectivity and can be applied for the separation of many analytes of pharmaceutical interest for which enantioseparation by CE with another CS systems can be problematic. Usually in a dual CD system an anionic CD is used together with a neutral one, but there are situations when the use of a cationic CD with a neutral one or the use of two neutral CDs or even two ionized CDs can be an efficient solution. In the current review we present general aspects of the use of dual CD systems in the analysis of pharmaceutical substances. Several examples of applications of the use of dual CD systems in the analysis of pharmaceuticals are selected and discussed. Theoretical aspects regarding the separation of enantiomers through simultaneous interaction with the two CSs are also explained. Finally, advantages, disadvantages, potential and new direction in this chiral analysis field are highlighted.
Keywords: capillary electrophoresis; chiral selectors; chiral separation; cyclodextrins; dual cyclodextrin system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
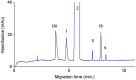
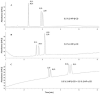
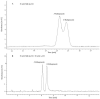
References
-
- Zhang Y., Yao S., Zeng H., Song H. Chiral separation of pharmaceuticals by high performance liquid chromatography. Curr. Pharm. Anal. 2010;6:114–130. doi: 10.2174/157341210791202636. - DOI
-
- He L., Beesley T.E. Applications of enantiomeric gas chromatography: A review. J. Liq. Chrom. Relat. Tech. 2005;28:1075–1114. doi: 10.1081/JLC-200052997. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

