RNA-Targeting Splicing Modifiers: Drug Development and Screening Assays
- PMID: 33919699
- PMCID: PMC8070285
- DOI: 10.3390/molecules26082263
RNA-Targeting Splicing Modifiers: Drug Development and Screening Assays
Abstract
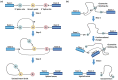
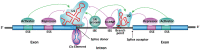
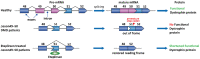
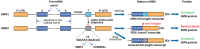
RNA splicing is an essential step in producing mature messenger RNA (mRNA) and other RNA species. Harnessing RNA splicing modifiers as a new pharmacological modality is promising for the treatment of diseases caused by aberrant splicing. This drug modality can be used for infectious diseases by disrupting the splicing of essential pathogenic genes. Several antisense oligonucleotide splicing modifiers were approved by the U.S. Food and Drug Administration (FDA) for the treatment of spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD). Recently, a small-molecule splicing modifier, risdiplam, was also approved for the treatment of SMA, highlighting small molecules as important warheads in the arsenal for regulating RNA splicing. The cellular targets of these approved drugs are all mRNA precursors (pre-mRNAs) in human cells. The development of novel RNA-targeting splicing modifiers can not only expand the scope of drug targets to include many previously considered "undruggable" genes but also enrich the chemical-genetic toolbox for basic biomedical research. In this review, we summarized known splicing modifiers, screening methods for novel splicing modifiers, and the chemical space occupied by the small-molecule splicing modifiers.
Keywords: RNA-targeting; alternative splicing; antisense oligonucleotide; high-throughput screening; small molecule; splicing modifier.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



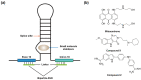




References
-
- Clemens P.R., Rao V.K., Connolly A.M., Harper A.D., Mah J.K., Smith E.C., McDonald C.M., Zaidman C.M., Morgenroth L.P., Osaki H., et al. Safety, tolerability, and efficacy of viltolarsen in boys with duchenne muscular dystrophy amenable to exon 53 skipping: A phase 2 randomized clinical trial. JAMA Neurol. 2020;77:982–991. doi: 10.1001/jamaneurol.2020.1264. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

