Detection of SARS-COV-2 Proteins Using an ELISA Test
- PMID: 33919728
- PMCID: PMC8070680
- DOI: 10.3390/diagnostics11040698
Detection of SARS-COV-2 Proteins Using an ELISA Test
Abstract
The coronavirus disease 2019 (COVID-19) global pandemic created an unprecedented public health emergency. Early recognition of an infected person and disruption of the transmission pathway are the keys to controlling this major public health threat around the world. The scientifically reliable screening method is an RT-PCR test that is performed on an ororhinopharyngeal swab in the laboratory. In the current severe SARS-CoV-2 pandemic, it is necessary to identify devices for rapid diagnosis to reduce the spread of the disease. The aim of this study was to provide a qualitative, rapid, sensitive, and specific method for a diagnosis of SARS-CoV-2 infection based on the recognition of specific antigens of the SARS-CoV-2 virus. The device was built by assembling commercially available and custom-made semi-finished products. The method was performed in environments outside the laboratory, i.e., "patient side," with an immediate chemocolorimetric response or with a digital reader using an ELISA method.
Keywords: COVID-19; ELISA; SARS-CoV-2; cytosalivary sampling.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


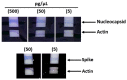
References
-
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 22 October 2020)];2020 Available online: https://covid19.who.int.
-
- WHO Coronavirus Disease (COVID-2019) Situation Report–51. [(accessed on 24 November 2020)];2020 Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- WHO Laboratory Diagnostics for Novel Coronavirus. [(accessed on 27 January 2021)];2020 Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

