CgEnd3 Regulates Endocytosis, Appressorium Formation, and Virulence in the Poplar Anthracnose Fungus Colletotrichum gloeosporioides
- PMID: 33919762
- PMCID: PMC8103510
- DOI: 10.3390/ijms22084029
CgEnd3 Regulates Endocytosis, Appressorium Formation, and Virulence in the Poplar Anthracnose Fungus Colletotrichum gloeosporioides
Abstract
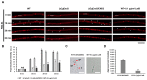
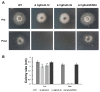
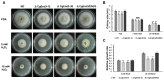
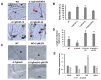
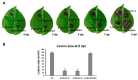
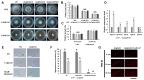
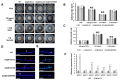
The hemibiotrophic ascomycete fungus Colletotrichum gloeosporioides is the causal agent of anthracnose on numerous plants, and it causes considerable economic losses worldwide. Endocytosis is an essential cellular process in eukaryotic cells, but its roles in C. gloeosporioides remain unknown. In our study, we identified an endocytosis-related protein, CgEnd3, and knocked it out via polyethylene glycol (PEG)-mediated protoplast transformation. The lack of CgEnd3 resulted in severe defects in endocytosis. C. gloeosporioides infects its host through a specialized structure called appressorium, and ΔCgEnd3 showed deficient appressorium formation, melanization, turgor pressure accumulation, penetration ability of appressorium, cellophane membrane penetration, and pathogenicity. CgEnd3 also affected oxidant adaptation and the expression of core effectors during the early stage of infection. CgEnd3 contains one EF hand domain and four calcium ion-binding sites, and it is involved in calcium signaling. A lack of CgEnd3 changed the responses to cell-wall integrity agents and fungicide fludioxonil. However, CgEnd3 regulated appressorium formation and endocytosis in a calcium signaling-independent manner. Taken together, these results demonstrate that CgEnd3 plays pleiotropic roles in endocytosis, calcium signaling, cell-wall integrity, appressorium formation, penetration, and pathogenicity in C. gloeosporioides, and it suggests that CgEnd3 or endocytosis-related genes function as promising antifungal targets.
Keywords: Colletotrichum gloeosporioides; anthracnose; appressorium; calcium signaling; endocytosis; pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Li Z., Liang Y.-M., Tian C. Characterization of the causal agent of poplar anthracnose occurring in the Beijing region. Mycotaxon. 2012;120:277–286. doi: 10.5248/120.277. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

