Impaired Ca2+ Sensitivity of a Novel GCAP1 Variant Causes Cone Dystrophy and Leads to Abnormal Synaptic Transmission Between Photoreceptors and Bipolar Cells
- PMID: 33919796
- PMCID: PMC8070792
- DOI: 10.3390/ijms22084030
Impaired Ca2+ Sensitivity of a Novel GCAP1 Variant Causes Cone Dystrophy and Leads to Abnormal Synaptic Transmission Between Photoreceptors and Bipolar Cells
Abstract
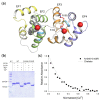
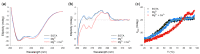
Guanylate cyclase-activating protein 1 (GCAP1) is involved in the shutdown of the phototransduction cascade by regulating the enzymatic activity of retinal guanylate cyclase via a Ca2+/cGMP negative feedback. While the phototransduction-associated role of GCAP1 in the photoreceptor outer segment is widely established, its implication in synaptic transmission to downstream neurons remains to be clarified. Here, we present clinical and biochemical data on a novel isolate GCAP1 variant leading to a double amino acid substitution (p.N104K and p.G105R) and associated with cone dystrophy (COD) with an unusual phenotype. Severe alterations of the electroretinogram were observed under both scotopic and photopic conditions, with a negative pattern and abnormally attenuated b-wave component. The biochemical and biophysical analysis of the heterologously expressed N104K-G105R variant corroborated by molecular dynamics simulations highlighted a severely compromised Ca2+-sensitivity, accompanied by minor structural and stability alterations. Such differences reflected on the dysregulation of both guanylate cyclase isoforms (RetGC1 and RetGC2), resulting in the constitutive activation of both enzymes at physiological levels of Ca2+. As observed with other GCAP1-associated COD, perturbation of the homeostasis of Ca2+ and cGMP may lead to the toxic accumulation of second messengers, ultimately triggering cell death. However, the abnormal electroretinogram recorded in this patient also suggested that the dysregulation of the GCAP1-cyclase complex further propagates to the synaptic terminal, thereby altering the ON-pathway related to the b-wave generation. In conclusion, the pathological phenotype may rise from a combination of second messengers' accumulation and dysfunctional synaptic communication with bipolar cells, whose molecular mechanisms remain to be clarified.
Keywords: GUCA1A; bipolar cells; calcium binding proteins; cone dystrophy; guanylate cyclase; neuronal calcium sensor; photoreceptors; phototransduction; retinal degeneration; synaptic transmission.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

