Pancreatic Neuroendocrine Neoplasms in Multiple Endocrine Neoplasia Type 1
- PMID: 33919851
- PMCID: PMC8070788
- DOI: 10.3390/ijms22084041
Pancreatic Neuroendocrine Neoplasms in Multiple Endocrine Neoplasia Type 1
Abstract
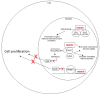
Pancreatic neuroendocrine tumors (pNETs) are a rare group of cancers accounting for about 1-2% of all pancreatic neoplasms. About 10% of pNETs arise within endocrine tumor syndromes, such as Multiple Endocrine Neoplasia type 1 (MEN1). pNETs affect 30-80% of MEN1 patients, manifesting prevalently as multiple microadenomas. pNETs in patients with MEN1 are particularly difficult to treat due to differences in their growth potential, their multiplicity, the frequent requirement of extensive surgery, the high rate of post-operative recurrences, and the concomitant development of other tumors. MEN1 syndrome is caused by germinal heterozygote inactivating mutation of the MEN1 gene, encoding the menin tumor suppressor protein. MEN1-related pNETs develop following the complete loss of function of wild-type menin. Menin is a key regulator of endocrine cell plasticity and its loss in these cells is sufficient for tumor initiation. Somatic biallelic loss of wild-type menin in the neuroendocrine pancreas presumably alters the epigenetic control of gene expression, mediated by histone modifications and DNA hypermethylation, as a driver of MEN1-associated pNET tumorigenesis. In this light, epigenetic-based therapies aimed to correct the altered DNA methylation, and/or histone modifications might be a possible therapeutic strategy for MEN1 pNETs, for whom standard treatments fail.
Keywords: MEN1 gene; Multiple Endocrine Neoplasia type 1 (MEN1); epigenetic factors; gene mutation; menin; pancreatic neuroendocrine tumors (pNETs).
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures
References
-
- Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

