Etiology of Clinical Community-Acquired Pneumonia in Swedish Children Aged 1-59 Months with High Pneumococcal Vaccine Coverage-The TREND Study
- PMID: 33919904
- PMCID: PMC8070909
- DOI: 10.3390/vaccines9040384
Etiology of Clinical Community-Acquired Pneumonia in Swedish Children Aged 1-59 Months with High Pneumococcal Vaccine Coverage-The TREND Study
Abstract
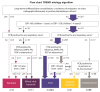
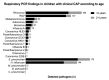
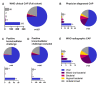
(1) Immunization with pneumococcal conjugate vaccines has decreased the burden of community-acquired pneumonia (CAP) in children and likely led to a shift in CAP etiology. (2) The Trial of Respiratory infections in children for ENhanced Diagnostics (TREND) enrolled children 1-59 months with clinical CAP according to the World Health Organization (WHO) criteria at Sachs' Children and Youth Hospital, Stockholm, Sweden. Children with rhonchi and indrawing underwent "bronchodilator challenge". C-reactive protein and nasopharyngeal PCR detecting 20 respiratory pathogens, were collected from all children. Etiology was defined according to an a priori defined algorithm based on microbiological, biochemical, and radiological findings. (3) Of 327 enrolled children, 107 (32%) required hospitalization; 91 (28%) received antibiotic treatment; 77 (24%) had a chest X-ray performed; and 60 (18%) responded to bronchodilator challenge. 243 (74%) episodes were classified as viral, 11 (3%) as mixed viral-bacterial, five (2%) as bacterial, two (0.6%) as atypical bacterial and 66 (20%) as undetermined etiology. After exclusion of children responding to bronchodilator challenge, the proportion of bacterial and mixed viral-bacterial etiology was 1% and 4%, respectively. (4) The novel TREND etiology algorithm classified the majority of clinical CAP episodes as of viral etiology, whereas bacterial etiology was uncommon. Defining CAP in children <5 years is challenging, and the WHO definition of clinical CAP is not suitable for use in children immunized with pneumococcal conjugate vaccines.
Keywords: World Health Organization; bacterial pneumonia; children; etiology; pneumococcal conjugate vaccines; respiratory infection; viral pneumonia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The impact of 13-valent pneumococcal conjugate vaccination on virus-associated community-acquired pneumonia in elderly: Exploratory analysis of the CAPiTA trial.Clin Microbiol Infect. 2018 Jul;24(7):764-770. doi: 10.1016/j.cmi.2017.10.006. Epub 2017 Oct 16. Clin Microbiol Infect. 2018. PMID: 29050992 Free PMC article. Clinical Trial.
-
The impact of pneumococcal conjugate vaccine on community-acquired pneumonia hospitalizations in children with comorbidity.Eur J Pediatr. 2017 Mar;176(3):337-342. doi: 10.1007/s00431-016-2843-2. Epub 2017 Jan 9. Eur J Pediatr. 2017. PMID: 28070670 Free PMC article.
-
Introducing a New Algorithm for Classification of Etiology in Studies on Pediatric Pneumonia: Protocol for the Trial of Respiratory Infections in Children for Enhanced Diagnostics Study.JMIR Res Protoc. 2019 Apr 26;8(4):e12705. doi: 10.2196/12705. JMIR Res Protoc. 2019. PMID: 31025954 Free PMC article.
-
Evaluation of vaccines for the prevention of pneumonia in children in developing countries.Epidemiol Rev. 1999;21(1):43-55. doi: 10.1093/oxfordjournals.epirev.a017987. Epidemiol Rev. 1999. PMID: 10520472 Review.
-
Community-acquired pneumonia in the post 13-valent pneumococcal conjugate vaccine era.Curr Opin Pediatr. 2016 Dec;28(6):786-793. doi: 10.1097/MOP.0000000000000428. Curr Opin Pediatr. 2016. PMID: 27755118 Review.
Cited by
-
Epidemiology of Respiratory Pathogens in Children with Severe Acute Respiratory Infection and Impact of the Multiplex PCR Film Array Respiratory Panel: A 2-Year Study.Int J Microbiol. 2021 Dec 31;2021:2276261. doi: 10.1155/2021/2276261. eCollection 2021. Int J Microbiol. 2021. PMID: 35003265 Free PMC article.
-
Profiling the nasopharyngeal Microbiome in patients with community-acquired pneumonia caused by Streptococcus pneumoniae: diagnostic challenges and ecological insights.Med Microbiol Immunol. 2025 Apr 10;214(1):19. doi: 10.1007/s00430-025-00828-0. Med Microbiol Immunol. 2025. PMID: 40208342 Free PMC article.
-
Novel Biomarkers Differentiating Viral from Bacterial Infection in Febrile Children: Future Perspectives for Management in Clinical Praxis.Children (Basel). 2021 Nov 20;8(11):1070. doi: 10.3390/children8111070. Children (Basel). 2021. PMID: 34828783 Free PMC article. Review.
-
Comparison between Short Therapy and Standard Therapy in Pediatric Patients Hospitalized with Urinary Tract Infection: A Single Center Retrospective Analysis.Children (Basel). 2022 Oct 28;9(11):1647. doi: 10.3390/children9111647. Children (Basel). 2022. PMID: 36360375 Free PMC article.
-
Utility of Rapid Nasopharyngeal Swab for Respiratory Pathogens in the Diagnosis of Viral Infections in Children Hospitalized with Fever: A Prospective Validation Study to Improve Antibiotic Use.Children (Basel). 2024 Feb 9;11(2):225. doi: 10.3390/children11020225. Children (Basel). 2024. PMID: 38397338 Free PMC article.
References
-
- Lindstrand A., Bennet R., Galanis I., Blennow M., Ask L.S., Dennison S.H., Rinder M.R., Eriksson M., Henriques-Normark B., Ortqvist A., et al. Sinusitis and pneumonia hospitalization after introduction of pneumococcal conjugate vaccine. Pediatrics. 2014;134:e1528–e1536. doi: 10.1542/peds.2013-4177. - DOI - PubMed
-
- Wahl B., O’Brien K.L., Greenbaum A., Majumder A., Liu L., Chu Y., Lukšić I., Nair H., McAllister D.A., Campbell H., et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15. Lancet Glob. Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. - DOI - PMC - PubMed
-
- Rhedin S., Lindstrand A., Hjelmgren A., Ryd-Rinder M., Ohrmalm L., Tolfvenstam T., Ortqvist A., Rotzen-Ostlund M., Zweygberg-Wirgart B., Henriques-Normark B., et al. Respiratory viruses associated with community-acquired pneumonia in children: Matched case-control study. Thorax. 2015;70:847–853. doi: 10.1136/thoraxjnl-2015-206933. - DOI - PubMed
-
- Zar H.J., Barnett W., Stadler A., Gardner-Lubbe S., Myer L., Nicol M.P. Aetiology of childhood pneumonia in a well vaccinated south african birth cohort: A nested case-control study of the drakenstein child health study. Lancet. Respir. Med. 2016;4:463–472. doi: 10.1016/S2213-2600(16)00096-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

