Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis
- PMID: 33919905
- PMCID: PMC8070970
- DOI: 10.3390/cancers13081880
Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis
Abstract
Background: Standard of care for locally advanced cervical cancer is chemoradiation and brachytherapy. The addition of adjuvant systemic treatment may improve overall survival. A systematic review and meta-analysis was conducted to summarize evidence on survival outcomes, treatment completion and toxicity.
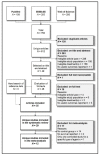
Methods: PubMed, EMBASE and Web of Science were systematically searched for relevant prospective and retrospective studies. Two authors independently selected studies, extracted data and assessed study quality. Pooled hazard ratios for survival endpoints were estimated using random effect models. Weighted averages of treatment completion and toxicity rates were calculated and compared by the Fisher exact test.
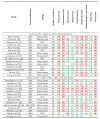
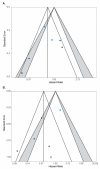
Results: The search returned 612 articles; 35 articles reporting on 29 different studies on adjuvant chemotherapy or immunotherapy were selected for systematic review. Twelve studies on an adjuvant platinum-pyrimidine antagonist or platinum-taxane were included for meta-analysis. The pooled hazard ratios for overall survival were 0.76 (99%CI: 0.43-1.34, p = 0.22) and 0.47 (99%CI: 0.12-1.86, p = 0.16) for the addition of, respectively, a platinum-pyrimidine antagonist or platinum-taxane to chemoradiation and brachytherapy. Completion rates were 82% (95%CI: 76-87%) for platinum-pyrimidine antagonist and 74% (95%CI: 63-85%) for platinum-taxane. Severe acute hematological and gastro-intestinal toxicities were significantly increased by adding adjuvant chemotherapy to chemoradiation and brachytherapy.
Conclusions: The addition of adjuvant platinum-pyrimidine antagonist or platinum-taxane after chemoradiation and brachytherapy does not significantly improve overall survival, while acute toxicity is significantly increased. These adjuvant treatment strategies can therefore not be recommended for unselected patients with locally advanced cervical cancer.
Keywords: adjuvant therapy; cervical cancer; chemotherapy; immunotherapy; meta-analysis; overall survival.
Conflict of interest statement
S.C. reports having received funding for research other than the current work from Varian International, Terry Fox Foundation, Department of Atomic Energy Clinical Trials Centre India, Department of Science and Technology India and International Atomic Energy Agency. R.N. reports having received funding for research other than the current work from the Dutch Cancer Foundation (KWF), Dutch Research Council (NWO), Elekta, Varian, Accuray and Merck Radiation Therapy Advisory Board Meeting on November 6th, 2020. The other others have declared no conflicts of interest.
Figures

References
-
- Ferlay: J., Ervik M., Lam F., Colombet M., Mery L., Pineros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Obser-vatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. [(accessed on 4 September 2020)];2018 Available online: https://gco.iarc.fr/today.
-
- Trimble E.L., Gius D., Harlan L.C. Impact of NCI Clinical Announcement upon use of chemoradiation for women with cervical cancer. J. Clin. Oncol. 2007;25:5537. doi: 10.1200/jco.2007.25.18_suppl.5537. - DOI
-
- Sturdza A., Pötter R., Fokdal L.U., Haie-Meder C., Tan L.T., Mazeron R., Petric P., Šegedin B., Jurgenliemk-Schulz I.M., Nomden C., et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. - DOI - PubMed
-
- Mahantshetty U., Krishnatry R., Hande V., Jamema S., Ghadi Y., Engineer R., Chopra S., Gurram L., Deshpande D., Shrviastava S. Magnetic Resonance Image Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer: An Experience From a Tertiary Cancer Center in a Low and Middle Income Countries Setting. Int. J. Radiat. Oncol. 2017;99:608–617. doi: 10.1016/j.ijrobp.2017.06.010. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

