Intravenous Vipera berus Venom-Specific Fab Fragments and Intramuscular Vipera ammodytes Venom-Specific F(ab')2 Fragments in Vipera ammodytes-Envenomed Patients
- PMID: 33919927
- PMCID: PMC8070888
- DOI: 10.3390/toxins13040279
Intravenous Vipera berus Venom-Specific Fab Fragments and Intramuscular Vipera ammodytes Venom-Specific F(ab')2 Fragments in Vipera ammodytes-Envenomed Patients
Abstract
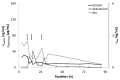
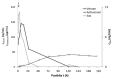
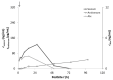
Vipera ammodytes (V. ammodytes) is the most venomous European viper. The aim of this study was to compare the clinical efficacy and pharmacokinetic values of intravenous Vipera berus venom-specific (paraspecific) Fab fragments (ViperaTAb) and intramuscular V. ammodytes venom-specific F(ab')2 fragments (European viper venom antiserum, also called "Zagreb" antivenom) in V.ammodytes-envenomed patients. This was a prospective study of V.ammodytes-envenomed patients that were treated intravenously with ViperaTAb or intramuscularly with European viper venom antiserum that was feasible only due to the unique situation of an antivenom shortage. The highest venom concentration, survival, length of hospital stay and adverse reactions did not differ between the groups. Patients treated with intravenous Fab fragments were sicker, with significantly more rhabdomyolysis and neurotoxicity. The kinetics of Fab fragments after one or more intravenous applications matched better with the venom concentration in the early phase of envenomation compared to F(ab')2 fragments that were given intramuscularly only on admission. F(ab')2 fragments given intramuscularly had 25-fold longer apparent total body clearance and 14-fold longer elimination half-time compared to Fab fragments given intravenously (2 weeks vs. 24 h, respectively). In V.ammodytes-envenomed patients, the intramuscular use of specific F(ab')2 fragments resulted in a slow rise of antivenom serum concentration that demanded their early administration but without the need for additional doses for complete resolution of all clinical signs of envenomation. Intravenous use of paraspecific Fab fragments resulted in the immediate rise of antivenom serum concentration that enabled their use according to the clinical progress, but multiple doses might be needed for efficient therapy of thrombocytopenia due to venom recurrence, while the progression of rhabdomyolysis and neurotoxic effects of the venom could not be prevented.
Keywords: European viper venom antiserum; F(ab’)2 fragments; Fab fragments; V. ammodytes; ViperaTAb; nose-horned viper; pharmacokinetics; “Zagreb” antivenom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mallow D., Ludwig D., Nilson G. True Vipers: Natural History and Toxinology of Old-World Vipers. Krieger Publishing Company; Malabar, FL, USA: 2003. p. 359.
-
- Latinović Z., Leonardi A., Šribar J., Sajevic T., Žužek M.C., Frangež R., Halassy B., Trampuš-Bakija A., Pungerčar J., Križaj I. Venomics of Vipera berus berus to explain differences in pathology elicited by Vipera ammodytes ammodytes envenomation: Therapeutic implications. J. Proteom. 2016;146:34–47. doi: 10.1016/j.jprot.2016.06.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

