Embryonic Origin and Subclonal Evolution of Tumor-Associated Macrophages Imply Preventive Care for Cancer
- PMID: 33919979
- PMCID: PMC8071014
- DOI: 10.3390/cells10040903
Embryonic Origin and Subclonal Evolution of Tumor-Associated Macrophages Imply Preventive Care for Cancer
Abstract
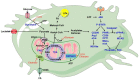
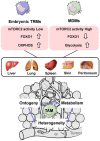
Macrophages are widely distributed in tissues and function in homeostasis. During cancer development, tumor-associated macrophages (TAMs) dominatingly support disease progression and resistance to therapy by promoting tumor proliferation, angiogenesis, metastasis, and immunosuppression, thereby making TAMs a target for tumor immunotherapy. Here, we started with evidence that TAMs are highly plastic and heterogeneous in phenotype and function in response to microenvironmental cues. We pointed out that efforts to tear off the heterogeneous "camouflage" in TAMs conduce to target de facto protumoral TAMs efficiently. In particular, several fate-mapping models suggest that most tissue-resident macrophages (TRMs) are generated from embryonic progenitors, and new paradigms uncover the ontogeny of TAMs. First, TAMs from embryonic modeling of TRMs and circulating monocytes have distinct transcriptional profiling and function, suggesting that the ontogeny of TAMs is responsible for the functional heterogeneity of TAMs, in addition to microenvironmental cues. Second, metabolic remodeling helps determine the mechanism of phenotypic and functional characteristics in TAMs, including metabolic bias from macrophages' ontogeny in macrophages' functional plasticity under physiological and pathological conditions. Both models aim at dissecting the ontogeny-related metabolic regulation in the phenotypic and functional heterogeneity in TAMs. We argue that gleaning from the single-cell transcriptomics on subclonal TAMs' origins may help understand the classification of TAMs' population in subclonal evolution and their distinct roles in tumor development. We envision that TAM-subclone-specific metabolic reprogramming may round-up with future cancer therapies.
Keywords: cancer target therapy; heterogeneity; metabolism; origins; subclonal evolution; therapy-resistant; tumor-associated macrophages (TAMs).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. - DOI - PMC - PubMed
-
- Bain C.C., Bravo-Blas A., Scott C.L., Perdiguero E.G., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. - DOI - PMC - PubMed
-
- Tamoutounour S., Guilliams M., Sanchis F.M., Liu H., Terhorst D., Malosse C., Pollet E., Ardouin L., Luche H., Sanchez C., et al. Origins and Functional Specialization of Macrophages and of Conventional and Monocyte-Derived Dendritic Cells in Mouse Skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

