Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives
- PMID: 33919988
- PMCID: PMC8071040
- DOI: 10.3390/jcm10081685
Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives
Abstract
Ewing sarcoma, a highly aggressive bone and soft-tissue cancer, is considered a prime example of the paradigms of a translocation-positive sarcoma: a genetically rather simple disease with a specific and neomorphic-potential therapeutic target, whose oncogenic role was irrefutably defined decades ago. This is a disease that by definition has micrometastatic disease at diagnosis and a dismal prognosis for patients with macrometastatic or recurrent disease. International collaborations have defined the current standard of care in prospective studies, delivering multiple cycles of systemic therapy combined with local treatment; both are associated with significant morbidity that may result in strong psychological and physical burden for survivors. Nevertheless, the combination of non-directed chemotherapeutics and ever-evolving local modalities nowadays achieve a realistic chance of cure for the majority of patients with Ewing sarcoma. In this review, we focus on the current standard of diagnosis and treatment while attempting to answer some of the most pressing questions in clinical practice. In addition, this review provides scientific answers to clinical phenomena and occasionally defines the resulting translational studies needed to overcome the hurdle of treatment-associated morbidities and, most importantly, non-survival.
Keywords: EWSR1-FLI1; chromosomal translocation; ewing sarcoma; fusion protein; limb salvage; metastasis; small round cell sarcoma; splicing; transcription.
Conflict of interest statement
J.A.T. is a co-founder of Oncternal Therapeutics, Inc., which is developing TK216. He holds founder equity and is a scientific consultant. S.G.D. has received consulting fees from Loxo Oncology and Bayer and travel expenses from Loxo Oncology and Salarius Pharmaceuticals. All other authors declare no conflict of interest.
Figures


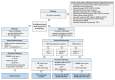


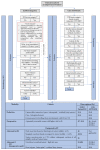

References
-
- Anderton J., Moroz V., Marec-Berard P., Gaspar N., Laurence V., Martin-Broto J., Sastre A., Gelderblom H., Owens C., Kaiser S., et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours-EURO EWING 2012 Protocol. Trials. 2020;21:96. doi: 10.1186/s13063-019-4026-8. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

