Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway
- PMID: 33919990
- PMCID: PMC8071060
- DOI: 10.3390/ijms22084056
Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway
Abstract
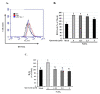
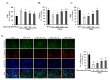
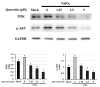
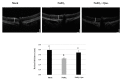
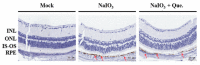
Age-related macular degeneration (AMD) leads to gradual central vision loss and is the third leading cause of irreversible blindness worldwide. The underlying mechanisms for this progressive neurodegenerative disease remain unclear and there is currently no preventive treatment for dry AMD. Sodium iodate (NaIO3) has been reported to induce AMD-like retinal pathology in mice. We established a mouse model for AMD to evaluate the effects of quercetin on NaIO3-induced retinal apoptosis, and to investigate the pertinent underlying mechanisms. Our in vitro results indicated that quercetin protected human retinal pigment epithelium (ARPE-19) cells from NaIO3-induced apoptosis by inhibiting reactive oxygen species production and loss of mitochondrial membrane potential as detected by Annexin V-FITC/PI flow cytometry. We also evaluated the relative expression of proteins in the apoptosis pathway. Quercetin downregulated the protein expressions of Bax, cleaved caspase-3, and cleaved PARP and upregulated the expression of Bcl-2 through reduced PI3K and pAKT expressions. Furthermore, our in vivo results indicated that quercetin improved retinal deformation and increased the thickness of both the outer nuclear layer and inner nuclear layer, whereas the expression of caspase-3 was inhibited. Taken together, these results demonstrate that quercetin could protect retinal pigment epithelium and the retina from NaIO3-induced cell apoptosis via reactive oxygen species-mediated mitochondrial dysfunction, involving the PI3K/AKT signaling pathway. This suggests that quercetin has the potential to prevent and delay AMD and other retinal diseases involving NaIO3-mediated apoptosis.
Keywords: age-related macular degeneration; apoptosis; human retinal pigment epithelium; mitochondrial membrane potential; quercetin; sodium iodate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Klein R., Myers C.E., Buitendijk G.H., Rochtchina E., Gao X., de Jong P.T., Sivakumaran T.A., Burlutsky G., McKean-Cowdin R., Hofman A., et al. Lipids, lipid genes, and incident age-related macular degeneration: The three continent age-related macular degeneration consortium. Am. J. Ophthalmol. 2014;158:513–524.e3. doi: 10.1016/j.ajo.2014.05.027. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

