A Yarrowia lipolytica Strain Engineered for Pyomelanin Production
- PMID: 33920006
- PMCID: PMC8071058
- DOI: 10.3390/microorganisms9040838
A Yarrowia lipolytica Strain Engineered for Pyomelanin Production
Abstract
The yeast Yarrowia lipolytica naturally produces pyomelanin. This pigment accumulates in the extracellular environment following the autoxidation and polymerization of homogentisic acid, a metabolite derived from aromatic amino acids. In this study, we used a chassis strain optimized to produce aromatic amino acids for the de novo overproduction of pyomelanin. The gene 4HPPD, which encodes an enzyme involved in homogentisic acid synthesis (4-hydroxyphenylpyruvic acid dioxygenase), was characterized and overexpressed in the chassis strain with up to three copies, leading to pyomelanin yields of 4.5 g/L. Homogentisic acid is derived from tyrosine. When engineered strains were grown in a phenylalanine-supplemented medium, pyomelanin production increased, revealing that the yeast could convert phenylalanine to tyrosine, or that the homogentisic acid pathway is strongly induced by phenylalanine.
Keywords: Yarrowia lipolytica; aromatic amino acids; chassis strain; pyomelanin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

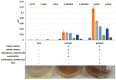



References
-
- Liu Y.-C., Chen S.-M., Liu J.-H., Hsu H.-W., Lin H.-Y., Chen S.-Y. Mechanical and photo-fragmentation processes for nanonization of melanin to improve its efficacy in protecting cells from reactive oxygen species stress. J. Appl. Phys. 2015;117:064701. doi: 10.1063/1.4907997. - DOI
-
- Ahn S.-Y., Choi M., Jeong D.-W., Park S., Park H., Jang K.-S., Choi K.-Y. Synthesis and chemical composition analysis of protocatechualdehyde-based novel melanin dye by 15T FT-ICR: High dyeing performance on soft contact lens. Dyes Pigments. 2019;160:546–554. doi: 10.1016/j.dyepig.2018.08.058. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

