Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal
- PMID: 33920057
- PMCID: PMC8071047
- DOI: 10.3390/microorganisms9040842
Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal
Abstract
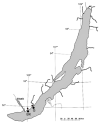
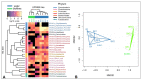
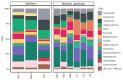
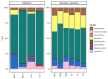
The diversity of aerobic anoxygenic phototrophs (AAPs) and rhodopsin-containing bacteria in the surface microlayer, water column, and epilithic biofilms of Lake Baikal was studied for the first time, employing pufM and rhodopsin genes, and compared to 16S rRNA diversity. We detected pufM-containing Alphaproteobacteria (orders Rhodobacterales, Rhizobiales, Rhodospirillales, and Sphingomonadales), Betaproteobacteria (order Burkholderiales), Gemmatimonadetes, and Planctomycetes. Rhodobacterales dominated all the studied biotopes. The diversity of rhodopsin-containing bacteria in neuston and plankton of Lake Baikal was comparable to other studied water bodies. Bacteroidetes along with Proteobacteria were the prevailing phyla, and Verrucomicrobia and Planctomycetes were also detected. The number of rhodopsin sequences unclassified to the phylum level was rather high: 29% in the water microbiomes and 22% in the epilithon. Diversity of rhodopsin-containing bacteria in epilithic biofilms was comparable with that in neuston and plankton at the phyla level. Unweighted pair group method with arithmetic mean (UPGMA) and non-metric multidimensional scaling (NMDS) analysis indicated a distinct discrepancy between epilithon and microbial communities of water (including neuston and plankton) in the 16S rRNA, pufM and rhodopsin genes.
Keywords: Lake Baikal; aerobic anoxygenic phototrophs; epilithon; neuston; plankton; pufM; rhodopsin; rhodopsin-containing bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Epilithic Biofilms in Lake Baikal: Screening and Diversity of PKS and NRPS Genes in the Genomes of Heterotrophic Bacteria.Pol J Microbiol. 2018;67(4):501-516. doi: 10.21307/pjm-2018-060. Pol J Microbiol. 2018. PMID: 30550237 Free PMC article.
-
[Taxonomic Composition of Lake Baikal Bacterioneuston Communities].Mikrobiologiia. 2017 Mar-Apr;86(2):229-38. Mikrobiologiia. 2017. PMID: 30299661 Russian.
-
Novel acsF Gene Primers Revealed a Diverse Phototrophic Bacterial Population, Including Gemmatimonadetes, in Lake Taihu (China).Appl Environ Microbiol. 2016 Aug 30;82(18):5587-94. doi: 10.1128/AEM.01063-16. Print 2016 Sep 15. Appl Environ Microbiol. 2016. PMID: 27401973 Free PMC article.
-
High bacterial diversity in epilithic biofilms of oligotrophic mountain lakes.Microb Ecol. 2012 Nov;64(4):860-9. doi: 10.1007/s00248-012-0072-4. Epub 2012 May 24. Microb Ecol. 2012. PMID: 22622765
-
A Metagenomic and Amplicon Sequencing Combined Approach Reveals the Best Primers to Study Marine Aerobic Anoxygenic Phototrophs.Microb Ecol. 2023 Oct;86(3):2161-2172. doi: 10.1007/s00248-023-02220-y. Epub 2023 May 6. Microb Ecol. 2023. PMID: 37148309 Free PMC article. Review.
Cited by
-
The Effects of a High Concentration of Dissolved Oxygen on Actinobacteria from Lake Baikal.Metabolites. 2023 Jul 7;13(7):830. doi: 10.3390/metabo13070830. Metabolites. 2023. PMID: 37512537 Free PMC article.
-
Diversity dynamics of aerobic anoxygenic phototrophic bacteria in a freshwater lake.Environ Microbiol Rep. 2023 Feb;15(1):60-71. doi: 10.1111/1758-2229.13131. Epub 2022 Dec 12. Environ Microbiol Rep. 2023. PMID: 36507772 Free PMC article.
-
Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia.Microorganisms. 2024 Nov 28;12(12):2450. doi: 10.3390/microorganisms12122450. Microorganisms. 2024. PMID: 39770653 Free PMC article.
-
Bacterial Metabolic Potential in Response to Climate Warming Alters the Decomposition Process of Aquatic Plant Litter-In Shallow Lake Mesocosms.Microorganisms. 2022 Jun 30;10(7):1327. doi: 10.3390/microorganisms10071327. Microorganisms. 2022. PMID: 35889044 Free PMC article.
-
Intestinal Microbiota Differences in Litopenaeus vannamei Shrimp between Greenhouse and Aquaponic Rearing.Life (Basel). 2023 Feb 14;13(2):525. doi: 10.3390/life13020525. Life (Basel). 2023. PMID: 36836882 Free PMC article.
References
-
- Yurkov V., Csotonyi J.T. New light on aerobic anoxygenic phototrophs. In: Hunter C.N., Daldal F., Thurnauer M.C., Beatty J.T., editors. The Purple Phototrophic Bacteria. Advances in PhotoSynthesis and Respiration. Volume 28. Springer; Dordrecht, The Netherlands: 2009. pp. 31–55. - DOI
-
- Yurkov V.V., Hughes E. Chapter eleven—Genes associated with the peculiar phenotypes of the aerobic anoxygenic phototrophs. Adv. Bot. Res. 2013;66:327–358. doi: 10.1016/B978-0-12-397923-0.00011-4. - DOI
-
- Yurkov V., Hughes E. Aerobic anoxygenic phototrophs: Four decades of mystery. In: Hallenbeck P., editor. Modern Topics in the Phototrophic Prokaryotes. Springer; Cham, Switzerland: 2017. pp. 193–214. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

