Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream
- PMID: 33920061
- PMCID: PMC8071103
- DOI: 10.3390/pharmaceutics13040555
Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream
Abstract
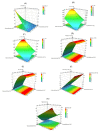
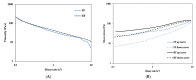
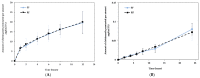
Semisolids constitute a significant proportion of topical pharmaceutical dosage forms available on the market, with creams being considered profitable systems for releasing active substances into the skin. This work aimed at the development of a generic Clotrimazole topical cream, based on the assumptions that assist the development of such formulations. First, the critical parameters to obtain a final formulation as similar as possible to the reference product were defined. Then, the percentages of cetyl palmitate and octyldodecanol were identified as critical variables and chosen for optimization in further studies. A "quality by design" approach was then used to identify the effect of process variability on the structural and functional similarity (Q3) of the generic product qualitatively (Q1) and quantitatively (Q2). A two-factor central composite orthogonal design was applied and eleven different formulations were developed and subjected to physicochemical characterization and product performance studies. The results were used to estimate the influence of the two variables in the variation of the responses, and to determine the optimum point of the tested factors, using a design space approach. Finally, an optimized formulation was obtained and analysed in parallel with the reference. The obtained results agreed with the prediction of the chemometric analysis, validating the reliability of the developed multivariate models. The in vitro release and permeation results were similar for the reference and the generic formulations, supporting the importance of interplaying microstructure properties with product performance and stability. Lastly, based on quality targets and response constraints, optimal working conditions were successfully achieved.
Keywords: generic medicine; pharmaceutical development; quality by design; rheology; topical delivery system.
Conflict of interest statement
The authors declare no conflict of interest. Daniel Arranca and Sara Raposo are the employees of The Laboratório Edol—Produtos Farmacêuticos, S.A. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Drilling down the bioequivalence assessment of topical antifungal products: Microstructure and release.Eur J Pharm Biopharm. 2023 Apr;185:94-106. doi: 10.1016/j.ejpb.2023.02.006. Epub 2023 Feb 19. Eur J Pharm Biopharm. 2023. PMID: 36806630
-
Development of performance matrix for generic product equivalence of acyclovir topical creams.Int J Pharm. 2014 Nov 20;475(1-2):110-22. doi: 10.1016/j.ijpharm.2014.07.034. Epub 2014 Jul 30. Int J Pharm. 2014. PMID: 25089511
-
Q1 and Q2 selection, Q3, IVRT, IVPT, pharmacokinetic and pharmacodynamic evaluation of topical generic product.Drug Dev Ind Pharm. 2025 Jun;51(6):555-565. doi: 10.1080/03639045.2025.2486487. Epub 2025 Apr 7. Drug Dev Ind Pharm. 2025. PMID: 40176255
-
Topical Semisolid Products-Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties.Pharmaceutics. 2022 Nov 17;14(11):2487. doi: 10.3390/pharmaceutics14112487. Pharmaceutics. 2022. PMID: 36432678 Free PMC article. Review.
-
Regulatory Requirements for the Development of Second-Entry Semisolid Topical Products in the European Union.Pharmaceutics. 2023 Feb 10;15(2):601. doi: 10.3390/pharmaceutics15020601. Pharmaceutics. 2023. PMID: 36839924 Free PMC article. Review.
Cited by
-
Understand the Stabilization Engineering of Ascorbic Acid, Mapping the Scheme for Stabilization, and Advancement.AAPS PharmSciTech. 2024 Jul 11;25(6):159. doi: 10.1208/s12249-024-02882-y. AAPS PharmSciTech. 2024. PMID: 38987438
-
Formulation and Evaluation of the In Vitro Performance of Topical Dermatological Products Containing Diclofenac Sodium.Pharmaceutics. 2022 Sep 7;14(9):1892. doi: 10.3390/pharmaceutics14091892. Pharmaceutics. 2022. PMID: 36145640 Free PMC article.
-
Reconstructed Human Epidermis: An Alternative Approach for In Vitro Bioequivalence Testing of Topical Products.Pharmaceutics. 2022 Jul 26;14(8):1554. doi: 10.3390/pharmaceutics14081554. Pharmaceutics. 2022. PMID: 35893811 Free PMC article.
-
Impact of Time on Parameters for Assessing the Microstructure Equivalence of Topical Products: Diclofenac 1% Emulsion as a Case Study.Pharmaceutics. 2024 Jun 1;16(6):749. doi: 10.3390/pharmaceutics16060749. Pharmaceutics. 2024. PMID: 38931871 Free PMC article.
-
Optimization of LCD-Based 3D Printing for the Development of Clotrimazole-Coated Microneedle Systems.Materials (Basel). 2025 Mar 31;18(7):1580. doi: 10.3390/ma18071580. Materials (Basel). 2025. PMID: 40271758 Free PMC article.
References
-
- Council of Europe . European Pharmacopoeia 8. Council of Europe; Strasbourg, France: 2014. European Directorate for the Quality of Medicines and Healthcare.
-
- Hornecker J.R. Generic Drugs: History, Approval Process, and Current Challenges. US Pharm. Generic Drug Rev. Suppl. 2009;34:26–30.
-
- European Medicines Agency . Draft Guideline on Quality and Equivalence of Topical Products. EMA; Amsterdam, The Netherlands: 2018.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

