Combination of Niraparib, Cisplatin and Twist Knockdown in Cisplatin-Resistant Ovarian Cancer Cells Potentially Enhances Synthetic Lethality through ER-Stress Mediated Mitochondrial Apoptosis Pathway
- PMID: 33920140
- PMCID: PMC8070209
- DOI: 10.3390/ijms22083916
Combination of Niraparib, Cisplatin and Twist Knockdown in Cisplatin-Resistant Ovarian Cancer Cells Potentially Enhances Synthetic Lethality through ER-Stress Mediated Mitochondrial Apoptosis Pathway
Abstract
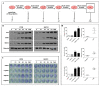
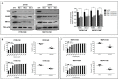
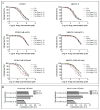
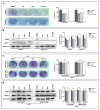
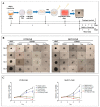
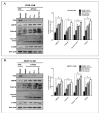
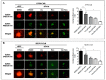
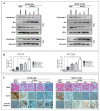
Poly (ADP-ribose) polymerase 1 inhibitors (PARPi) are used to treat recurrent ovarian cancer (OC) patients due to greater survival benefits and minimal side effects, especially in those patients with complete or partial response to platinum-based chemotherapy. However, acquired resistance of platinum-based chemotherapy leads to the limited efficacy of PARPi monotherapy in most patients. Twist is recognized as a possible oncogene and contributes to acquired cisplatin resistance in OC cells. In this study, we show how Twist knockdown cisplatin-resistant (CisR) OC cells blocked DNA damage response (DDR) to sensitize these cells to a concurrent treatment of cisplatin as a platinum-based chemotherapy agent and niraparib as a PARPi on in vitro two-dimensional (2D) and three-dimensional (3D) cell culture. To investigate the lethality of PARPi and cisplatin on Twist knockdown CisR OC cells, two CisR cell lines (OV90 and SKOV3) were established using step-wise dose escalation method. In addition, in vitro 3D spheroidal cell model was generated using modified hanging drop and hydrogel scaffolds techniques on poly-2-hydroxylethly methacrylate (poly-HEMA) coated plates. Twist expression was strongly correlated with the expression of DDR proteins, PARP1 and XRCC1 and overexpression of both proteins was associated with cisplatin resistance in OC cells. Moreover, combination of cisplatin (Cis) and niraparib (Nira) produced lethality on Twist-knockdown CisR OC cells, according to combination index (CI). We found that Cis alone, Nira alone, or a combination of Cis+Nira therapy increased cell death by suppressing DDR proteins in 2D monolayer cell culture. Notably, the combination of Nira and Cis was considerably effective against 3D-cultures of Twist knockdown CisR OC cells in which Endoplasmic reticulum (ER) stress is upregulated, leading to initiation of mitochondrial-mediated cell death. In addition, immunohistochemically, Cis alone, Nira alone or Cis+Nira showed lower ki-67 (cell proliferative marker) expression and higher cleaved caspase-3 (apoptotic marker) immuno-reactivity. Hence, lethality of PARPi with the combination of Cis on Twist knockdown CisR OC cells may provide an effective way to expand the therapeutic potential to overcome platinum-based chemotherapy resistance and PARPi cross resistance in OC.
Keywords: PARPi; Twist; cisplatin; cisplatin resistance; lethality; niraparib; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

