SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs
- PMID: 33920179
- PMCID: PMC8070527
- DOI: 10.3390/microorganisms9040793
SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs
Abstract
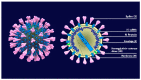
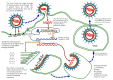
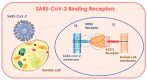
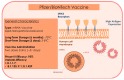
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), which is believed to have originated in China towards the end of November 2019, has now spread across the globe, causing a pandemic in 192 countries. The World Health Organization has called it the SARS-CoV-2 pandemic. Rapid dissemination of the virus occurs mainly through the saliva (Flügge's droplets) and aerosol, together with nasal and lachrymal passages. The literature associated with the recent advancement in terms of rapid diagnostics and SARS-CoV-2 vaccines has thoroughly studied the role of ACE2 receptors and Furin, as well as viral agent access into the host cell and its significant persistence at the level of the oral mucosa, which represents the main access to the virus. The purpose of this review was to underline the processes of SARS-CoV-2 infection mechanisms and novel breakthroughs in diagnostics and vaccines. Different technologies, such as the RT-PCR molecular test and the antigenic test, have been developed to identify subjects affected by the SARS-CoV-2 in order to improve the tracking of infection geographical diffusion. Novel rapid and highly sensitive diagnostic tests has been proposed for the detection of SARS-CoV-2 to improve the screening capability of suspected contagions. The strengthening of the vaccination campaign represents the most effective means to combat the SARS-CoV-2 infection and prevent severe manifestations of the virus-different classes of vaccines have been developed for this purpose. Further attention on the novel SARS-CoV-2 variant is necessary in order to verify the protection efficacy and virulence reduction of the infective agent in the recent vaccine campaign.
Keywords: ACE2; Cytokine Storm Syndrome (CSS); SARS-CoV-2 (COVID-19 pandemic); TMPRSS2; furin; microbiome; oral mucosa (salivary glands); vaccines.
Conflict of interest statement
The co-authors Angelo Michele Inchingolo, Andrea Ballini, Francesco Inchingolo, and Gianna Dipalma were involved in multicentred research at the Phan Chau Trinh University in Vietnam and the University of Bari Aldo Moro in Italy to develop diagnostic procedures for multiple detection of pandemic Coronaviridae family viruses. Patent number (s): 102020000011701 (Italy), EP20197796.4 (Europe), 17/034,407 (USA), PT20202942-FF-P-HK (Hong Kong). The other authors declare no conflict of interest in the present investigation.
Figures





References
-
- COVID-19 Map. [(accessed on 4 January 2021)]; Available online: https://coronavirus.jhu.edu/map.html.
-
- Balzanelli M.G., Distratis P., Aityan S.K., Amatulli F., Catucci O., Cefalo A., De Michele A., Dipalma G., Inchingolo F., Lazzaro R., et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets. 2021;21:1. doi: 10.2174/1871530321666210127141945. - DOI - PubMed
-
- Balzanelli M.G., Distratis P., Catucci O., Amatulli F., Cefalo A., Lazzaro R., Aityan K.S., Dalagni G., Nico A., de Michele A., et al. Clinical and diagnostic findings in COVID-19 patients: An original research from SG Moscati Hospital in Taranto Italy. J. Biol. Regul. Homeost. Agents. 2021;35:171–183. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

