Atherothrombosis in Acute Coronary Syndromes-From Mechanistic Insights to Targeted Therapies
- PMID: 33920201
- PMCID: PMC8070089
- DOI: 10.3390/cells10040865
Atherothrombosis in Acute Coronary Syndromes-From Mechanistic Insights to Targeted Therapies
Abstract
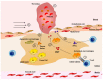
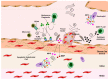
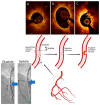
The atherothrombotic substrates for acute coronary syndromes (ACS) consist of plaque ruptures, erosions and calcified nodules, while the non-atherothrombotic etiologies, such as spontaneous coronary artery dissection, coronary artery spasm and coronary embolism are the rarer causes of ACS. The purpose of this comprehensive review is to (1) summarize the histopathologic insights into the atherothrombotic plaque subtypes in acute ACS from postmortem studies; (2) provide a brief overview of atherogenesis, while mainly focusing on the events that lead to plaque destabilization and disruption; (3) summarize mechanistic data from clinical studies that have used intravascular imaging, including high-resolution optical coherence tomography, to assess culprit plaque morphology and its underlying pathobiology, especially the newly described role of innate and adaptive immunity in ACS secondary to plaque erosion; (4) discuss the utility of intravascular imaging for effective treatment of patients presenting with ACS by percutaneous coronary intervention; and (5) discuss the opportunities that these mechanistic and imaging insights may provide for more individualized treatment of patients with ACS.
Keywords: acute coronary syndromes; calcified nodule; optical coherence tomography; percutaneous coronary intervention; plaque erosion; plaque rupture.
Conflict of interest statement
Madhavan is supported by an institutional grant by the National Heart, Lung, and Blood Institute of the US National Institutes of Health to Columbia University Irving Medical Center (T32 HL007854). The other authors declare no conflicts relevant to the content of this manuscript.
Figures

References
-
- Stary H.C., Chandler A.B., Glagov S., Guyton J.R., Insull W., Jr., Rosenfeld M.E., Schaffer S.A., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.CIR.89.5.2462. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

