Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives
- PMID: 33920281
- PMCID: PMC8070523
- DOI: 10.3390/molecules26082190
Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives
Abstract
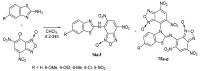
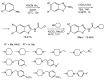
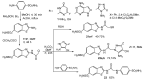
The review is devoted to modern trends in the chemistry of 2-amino and 2-mercapto substituted benzothiazoles covering the literature since 2015. The reviewed heterocycles belong to biologically active and industrially demanded compounds. Newly developed synthesis methods can be divided into conventional multistep processes and one-pot, atom economy procedures, realized using green chemistry principles and simple reagents. The easy functionalization of the 2-NH2 and 2-SH groups and the benzene ring of the benzothiazole moiety allows considering them as highly reactive building blocks for organic and organoelement synthesis, including the synthesis of pharmacologically active heterocycles. The review provides a summary of findings, which may be useful for developing new drugs and materials and new synthetic approaches and patterns of reactivity.
Keywords: 2-aminobenzothiazole; 2-mercaptobenzothiazole; benzothiazole; biological activity; reactivity; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



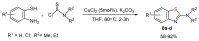
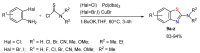
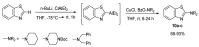


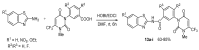

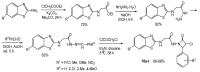




















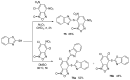

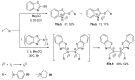

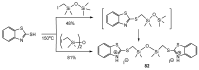
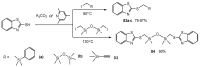

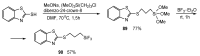
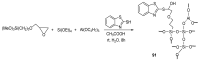

References
-
- Agarwal S., Gandhi D., Kalal P. Benzothiazole: A Versatile and Multitargeted Pharmacophore in the Field of Medicinal Chemistry. Lett. Org. Chem. 2017;14:729–742. doi: 10.2174/1570178614666170707160654. - DOI
-
- Liu X., Dong Z.-B. A Review on Domino Condensation/Cyclization Reactions for the Synthesis of 2-Substituted 1,3-Benzothiazole Derivatives. Eur. J. Org. Chem. 2020;4:408–419. doi: 10.1002/ejoc.201901502. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

