Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: The INCREASE Study
- PMID: 33920307
- PMCID: PMC8069749
- DOI: 10.3390/microorganisms9040798
Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: The INCREASE Study
Abstract
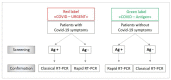
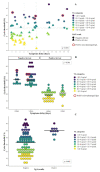
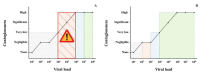
Following the Swiss Federal Office of Public Health (FOPH) authorization of the rapid antigen test (RAT), we implemented the use of the RAT in the emergency ward of our university hospital for patients' cohorting. RAT triaging in association with RT-PCR allowed us to promptly isolate positive patients and save resources. Among 532 patients, overall sensitivities were 48.3% for Exdia and 41.2% for Standard Q®, PanbioTM and BD Veritor™. All RATs exhibited specificity above 99%. Sensitivity increased to 74.6%, 66.2%, 66.2% and 64.8% for Exdia, Standard Q®, PanbioTM and BD Veritor™, respectively, for viral loads above 105 copies/mL, to 100%, 97.8%, 96.6% and 95.6% for viral loads above 106 copies/mL and 100% for viral loads above 107 copies/mL. Sensitivity was significantly higher for patients with symptoms onset within four days (74.3%, 69.2%, 69.2% and 64%, respectively) versus patients with the evolution of symptoms longer than four days (36.8%, 21.1%, 21.1% and 23.7%, respectively). Among COVID-19 asymptomatic patients, sensitivity was 33%. All Immunoglobulin-A-positive patients resulted negative for RAT. The RAT might represent a useful resource in selected clinical settings as a complementary tool in RT-PCR for rapid patient triaging, but the lower sensitivity, especially in late presenters and COVID-19 asymptomatic subjects, must be taken into account.
Keywords: COVID-19 diagnostic testing; SARS-CoV-2; emergency ward; health plan implementation; nucleocapsid protein; rapid antigen test.
Conflict of interest statement
Caruana, Kampouri, Kritikos, Opota, Foster, R. Brouillet, Senn, Lienhard, Egli, Pantaleo, Carron: no conflicts. Croxatto reports grants from Becton Dickinson outside the submitted work. Greub reports grants from Resistell, from Nittobo, outside the submitted work and he is the co-director of “JeuPro,” a start-up distributing the game Krobs, a card game about microbe transmission.
Figures


References
-
- WHO Rolling Updates on Coronavirus Disease (COVID-19) [(accessed on 25 January 2021)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a....
-
- ECDC COVID-19 Situation Update Worldwide, as of Week 2. [(accessed on 25 January 2021)];2021 Available online: https://covid19-country-overviews.ecdc.europa.eu/
-
- Adrian Egli R.L., Katia J., Gilbert G. Recommendation of the Swiss Society of Microbiology for usage of SARS-CoV-2 specific antigen tests. Pipette Swiss Lab. Med. 2020;6:18–20.
-
- FOPH Coronavirus: Tests. [(accessed on 25 January 2021)]; Available online: https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pa....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

