Circulating Tumor DNA Early Kinetics Predict Response of Metastatic Melanoma to Anti-PD1 Immunotherapy: Validation Study
- PMID: 33920470
- PMCID: PMC8069589
- DOI: 10.3390/cancers13081826
Circulating Tumor DNA Early Kinetics Predict Response of Metastatic Melanoma to Anti-PD1 Immunotherapy: Validation Study
Abstract
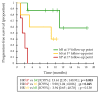
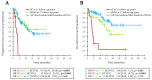
The ability of early (first weeks of treatment) ctDNA kinetics to identify primary resistance to anti-PD1 immunotherapies was evaluated with a validation cohort of 49 patients treated with anti-PD1 for metastatic BRAF or NRAS-mutated melanoma, alone and pooled with the 53 patients from a previously described derivation cohort. BRAF or NRAS mutations were quantified on plasma DNA by digital PCR at baseline and after two or four weeks of treatment. ctDNA kinetics were interpreted according to pre-established biological response criteria. A biological progression (bP, i.e., a significant increase in ctDNA levels) at week two or week four was associated with a lack of benefit from anti-PD1 (4-month PFS = 0%; 1-year OS = 13%; n = 12/102). Patients without initial bP had significantly better PFS and OS (4-month PFS = 78%; 1-year OS = 73%; n = 26/102), as did patients whose ctDNA kinetics were not evaluable, due to low/undetectable baseline ctDNA (4-month PFS = 80%; 1-year OS = 81%; n = 64/102). ctDNA detection at first-line anti-PD1 initiation was an independent prognostic factor for OS and PFS in multivariate analysis. Overall, early ctDNA quantitative monitoring may allow the detection of primary resistances of metastatic melanoma to anti-PD1 immunotherapies.
Keywords: anti-PD1; cell-free DNA; circulating tumor DNA; criteria; digital PCR; follow-up; immunotherapy; melanoma; metastatic melanoma; monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Larkin J., Minor D., D’Angelo S., Neyns B., Smylie M., Miller W.H., Gutzmer R., Linette G., Chmielowski B., Lao C.D., et al. Overall Survival in Patients with Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:383–390. doi: 10.1200/JCO.2016.71.8023. - DOI - PMC - PubMed
-
- Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C., Hodi F.S., Schachter J., Pavlick A.C., Lewis K.D., et al. Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

