Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms
- PMID: 33920514
- PMCID: PMC8072982
- DOI: 10.3390/jcm10081741
Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
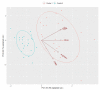
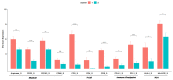
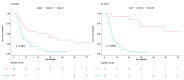
High-grade Gastroenteropancreatic Neuroendocrine neoplasms (H-NENs) comprehend well-differentiated tumors (NET G3) and poorly differentiated carcinomas (NEC) with proliferative activity indexes as mitotic count (MC) >20 mitoses/10 HPF and Ki-67 >20%. At present, no specific therapy for H-NENs exists and the several evidences of microenvironment involvement in their pathogenesis pave the way for tailored therapies. Forty-five consecutive cases, with available information about T-cell, immune, and non-immune markers, from surgical pathology and clinical databases of 2 Italian institutions were immunostained for Arginase, CD33, CD163 and CD66 myeloid markers. The association between features was assessed by Spearman's correlation coefficient. A unsupervised K-means algorithm was used to identify clusters of patients according to inputs of microenvironment features and the relationship between clusters and clinicopathological features, including cancer-specific survival (CSS), was analyzed. The H-NEN population was composed of 6 (13.3%) NET G3 and 39 (86.7%) NEC. Overall, significant positive associations were found between myeloid (CD33, CD163 and Arginase) and T/immune markers (CD3, CD4, CD8, PD-1 and HLA-I). Myeloid and T-cell markers CD3 and CD8 identified two clusters of patients from unsupervised K-means analysis. Cases grouped in cluster 1 with more myeloid infiltrates, T cell, HLA and expression of inhibitory receptors and ligands in the stroma (PD-1, PD-L1) had significantly better CSS than patients in cluster 2. Multivariable analysis showed that Ki-67 (>55 vs. <55, HR 8.60, CI 95% 2.61-28.33, p < 0.0001) and cluster (1 vs. 2, HR 0.43, CI 95% 0.20-0.93, p = 0.03) were significantly associated with survival. High grade gastroenteropancreatic neuroendocrine neoplasms can be further classified into two prognostic sub-populations of tumors driven by different tumor microenvironments and immune features able to generate the framework for evaluating new therapeutic strategies.
Keywords: gastroenteropancreatic neuroendocrine neoplasms; myeloid markers; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington M.K., Carneiro F., Cree I.A., The WHO Classification of Tumours Editorial Board The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. - DOI - PMC - PubMed
-
- Milione M., Maisonneuve P., Spada F., Pellegrinelli A., Spaggiari P., Albarello L., Pisa E., Barberis M., Vanoli A., Buzzoni R., et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: Morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104:85–93. doi: 10.1159/000445165. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

