Growth Restriction and Genomic Imprinting-Overlapping Phenotypes Support the Concept of an Imprinting Network
- PMID: 33920525
- PMCID: PMC8073901
- DOI: 10.3390/genes12040585
Growth Restriction and Genomic Imprinting-Overlapping Phenotypes Support the Concept of an Imprinting Network
Abstract
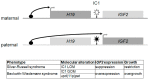
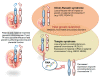
Intrauterine and postnatal growth disturbances are major clinical features of imprinting disorders, a molecularly defined group of congenital syndromes caused by molecular alterations affecting parentally imprinted genes. These genes are expressed monoallelically and in a parent-of-origin manner, and they have an impact on human growth and development. In fact, several genes with an exclusive expression from the paternal allele have been shown to promote foetal growth, whereas maternally expressed genes suppress it. The evolution of this correlation might be explained by the different interests of the maternal and paternal genomes, aiming for the conservation of maternal resources for multiple offspring versus extracting maximal maternal resources. Since not all imprinted genes in higher mammals show the same imprinting pattern in different species, the findings from animal models are not always transferable to human. Therefore, human imprinting disorders might serve as models to understand the complex regulation and interaction of imprinted loci. This knowledge is a prerequisite for the development of precise diagnostic tools and therapeutic strategies for patients affected by imprinting disorders. In this review we will specifically overview the current knowledge on imprinting disorders associated with growth retardation, and its increasing relevance in a personalised medicine direction and the need for a multidisciplinary therapeutic approach.
Keywords: Prader-Willi syndrome; Silver-Russell syndrome; differentially methylated regions; growth restriction; imprinted gene network; imprinting disorders; overgrowth; pseudoparahypoparathyreoidism; temple syndrome; transient neonatal diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci.Hum Mol Genet. 2009 Dec 15;18(24):4724-33. doi: 10.1093/hmg/ddp435. Epub 2009 Sep 14. Hum Mol Genet. 2009. PMID: 19755383
-
[Epigenetics, genomic imprinting and developmental disorders].Bull Acad Natl Med. 2010 Feb;194(2):287-97; discussion 297-300. Bull Acad Natl Med. 2010. PMID: 21166119 French.
-
Disturbed genomic imprinting and its relevance for human reproduction: causes and clinical consequences.Hum Reprod Update. 2020 Feb 28;26(2):197-213. doi: 10.1093/humupd/dmz045. Hum Reprod Update. 2020. PMID: 32068234 Review.
-
In-utero stress and mode of conception: impact on regulation of imprinted genes, fetal development and future health.Hum Reprod Update. 2019 Nov 5;25(6):777-801. doi: 10.1093/humupd/dmz025. Hum Reprod Update. 2019. PMID: 31633761
-
Genomic imprinting in the human placenta.Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S152-62. doi: 10.1016/j.ajog.2015.06.032. Am J Obstet Gynecol. 2015. PMID: 26428495 Review.
Cited by
-
Imprinting disorders.Nat Rev Dis Primers. 2023 Jun 29;9(1):33. doi: 10.1038/s41572-023-00443-4. Nat Rev Dis Primers. 2023. PMID: 37386011 Review.
-
Imprinted Grb10, encoding growth factor receptor bound protein 10, regulates fetal growth independently of the insulin-like growth factor type 1 receptor (Igf1r) and insulin receptor (Insr) genes.BMC Biol. 2024 May 30;22(1):127. doi: 10.1186/s12915-024-01926-w. BMC Biol. 2024. PMID: 38816743 Free PMC article.
-
Prenatal diagnosis of a silver-russell syndrome caused by 11p15 duplication and pedigree analysis.Front Genet. 2024 Dec 13;15:1465521. doi: 10.3389/fgene.2024.1465521. eCollection 2024. Front Genet. 2024. PMID: 39741906 Free PMC article.
-
Trans-acting genetic variants causing multilocus imprinting disturbance (MLID): common mechanisms and consequences.Clin Epigenetics. 2022 Mar 16;14(1):41. doi: 10.1186/s13148-022-01259-x. Clin Epigenetics. 2022. PMID: 35296332 Free PMC article.
-
DNA methylation analysis to differentiate reference, breed, and parent-of-origin effects in the bovine pangenome era.Gigascience. 2024 Jan 2;13:giae061. doi: 10.1093/gigascience/giae061. Gigascience. 2024. PMID: 39435573 Free PMC article.
References
-
- Gaillot-Durand L., Brioude F., Beneteau C., Le Breton F., Massardier J., Michon L., Devouassoux-Shisheboran M., Allias F. Placental Pathology in Beckwith-Wiedemann Syndrome According to Genotype/Epigenotype Subgroups. Fetal Pediatr. Pathol. 2018;37:387–399. doi: 10.1080/15513815.2018.1504842. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

