The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review
- PMID: 33920587
- PMCID: PMC8073160
- DOI: 10.3390/cells10040932
The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review
Abstract
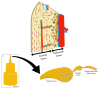
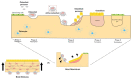
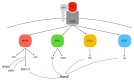
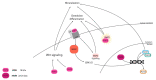
Bone is a hard-vascularized tissue, which renews itself continuously to adapt to the mechanical and metabolic demands of the body. The craniofacial area is prone to trauma and pathologies that often result in large bone damage, these leading to both aesthetic and functional complications for patients. The "gold standard" for treating these large defects is autologous bone grafting, which has some drawbacks including the requirement for a second surgical site with quantity of bone limitations, pain and other surgical complications. Indeed, tissue engineering combining a biomaterial with the appropriate cells and molecules of interest would allow a new therapeutic approach to treat large bone defects while avoiding complications associated with a second surgical site. This review first outlines the current knowledge of bone remodeling and the different signaling pathways involved seeking to improve our understanding of the roles of each to be able to stimulate or inhibit them. Secondly, it highlights the interesting characteristics of one growth factor in particular, FGF-2, and its role in bone homeostasis, before then analyzing its potential usefulness in craniofacial bone tissue engineering because of its proliferative, pro-angiogenic and pro-osteogenic effects depending on its spatial-temporal use, dose and mode of administration.
Keywords: FGF-2; angiogenesis; bone tissue engineering; mineralization; signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

