A Walk in the Memory, from the First Functional Approach up to Its Regulatory Role of Mitochondrial Bioenergetic Flow in Health and Disease: Focus on the Adenine Nucleotide Translocator
- PMID: 33920595
- PMCID: PMC8073645
- DOI: 10.3390/ijms22084164
A Walk in the Memory, from the First Functional Approach up to Its Regulatory Role of Mitochondrial Bioenergetic Flow in Health and Disease: Focus on the Adenine Nucleotide Translocator
Abstract
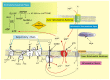
The mitochondrial adenine nucleotide translocator (ANT) plays the fundamental role of gatekeeper of cellular energy flow, carrying out the reversible exchange of ADP for ATP across the inner mitochondrial membrane. ADP enters the mitochondria where, through the oxidative phosphorylation process, it is the substrate of Fo-F1 ATP synthase, producing ATP that is dispatched from the mitochondrion to the cytoplasm of the host cell, where it can be used as energy currency for the metabolic needs of the cell that require energy. Long ago, we performed a method that allowed us to monitor the activity of ANT by continuously detecting the ATP gradually produced inside the mitochondria and exported in the extramitochondrial phase in exchange with externally added ADP, under conditions quite close to a physiological state, i.e., when oxidative phosphorylation takes place. More than 30 years after the development of the method, here we aim to put the spotlight on it and to emphasize its versatile applicability in the most varied pathophysiological conditions, reviewing all the studies, in which we were able to observe what really happened in the cell thanks to the use of the "ATP detecting system" allowing the functional activity of the ANT-mediated ADP/ATP exchange to be measured.
Keywords: ATP detecting system; adenine nucleotide translocator; disease; mitochondria; physiological role; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

