The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells
- PMID: 33920669
- PMCID: PMC8072566
- DOI: 10.3390/ph14040374
The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells
Abstract
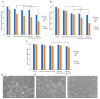
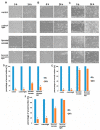
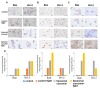
The research focused on the investigation of curcumin encapsulated in hydrogenated soy phosphatidylcholine liposomes and its increased photoactive properties in photodynamic therapy (PDT). The goal of this study was two-fold: to emphasize the role of a natural photoactive plant-based derivative in the liposomal formulation as an easily bioavailable, alternative photosensitizer (PS) for the use in PDT of skin malignancies. Furthermore, the goal includes to prove the decreased cytotoxicity of phototoxic agents loaded in liposomes toward normal skin cells. Research was conducted on melanoma (MugMel2), squamous cell carcinoma (SCC-25), and normal human keratinocytes (HaCaT) cell lines. The assessment of viability with MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) evaluated cell death after exposure to blue light irradiation after 4 h of pre-incubation with free and encapsulated curcumin. Additionally, the wound healing assay, flow cytometry, and immunocytochemistry to detect apoptosis were performed. The malignant cells revealed increased phototoxicity after the therapy in comparison to normal cells. Moreover, liposome curcumin-based photodynamic therapy showed an increased ratio of apoptotic and necrotic cells. The study also demonstrated that nanocurcumin significantly decreased malignant cell motility following PDT treatment. Acquired results suggest that liposomal formulation of a poor soluble natural compound may improve photosensitizing properties of curcumin-mediated PDT treatment in skin cancers and reduce toxicity in normal keratinocytes.
Keywords: curcumin; liposomes; melanoma; natural photosensitizer; normal keratinocytes; photodynamic therapy; skin cancer treatment; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hypericin-Based Photodynamic Therapy Displays Higher Selectivity and Phototoxicity towards Melanoma and Squamous Cell Cancer Compared to Normal Keratinocytes In Vitro.Int J Mol Sci. 2023 Nov 29;24(23):16897. doi: 10.3390/ijms242316897. Int J Mol Sci. 2023. PMID: 38069219 Free PMC article.
-
The Comparison of the Efficiency of Emodin and Aloe-Emodin in Photodynamic Therapy.Int J Mol Sci. 2022 Jun 3;23(11):6276. doi: 10.3390/ijms23116276. Int J Mol Sci. 2022. PMID: 35682955 Free PMC article.
-
Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes.Cancers (Basel). 2020 Nov 5;12(11):3278. doi: 10.3390/cancers12113278. Cancers (Basel). 2020. PMID: 33167593 Free PMC article.
-
Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer.Pharmaceutics. 2021 Sep 26;13(10):1562. doi: 10.3390/pharmaceutics13101562. Pharmaceutics. 2021. PMID: 34683855 Free PMC article. Review.
-
Nanostructural hybrid sensitizers for photodynamic therapy.Curr Pharm Des. 2012;18(18):2607-21. doi: 10.2174/138161212800492877. Curr Pharm Des. 2012. PMID: 22512446 Review.
Cited by
-
Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment.Pharmaceutics. 2023 Jun 12;15(6):1712. doi: 10.3390/pharmaceutics15061712. Pharmaceutics. 2023. PMID: 37376160 Free PMC article. Review.
-
Advances in Photodynamic Therapy Based on Nanotechnology and Its Application in Skin Cancer.Front Oncol. 2022 Mar 16;12:836397. doi: 10.3389/fonc.2022.836397. eCollection 2022. Front Oncol. 2022. PMID: 35372087 Free PMC article. Review.
-
Nanocurcumin in cancer treatment: a comprehensive systematic review.Discov Oncol. 2024 Oct 1;15(1):515. doi: 10.1007/s12672-024-01272-x. Discov Oncol. 2024. PMID: 39349709 Free PMC article. Review.
-
Non-melanoma skin cancers: physio-pathology and role of lipid delivery systems in new chemotherapeutic treatments.Neoplasia. 2022 Aug;30:100810. doi: 10.1016/j.neo.2022.100810. Epub 2022 May 29. Neoplasia. 2022. PMID: 35649306 Free PMC article. Review.
-
Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation.Pharmaceutics. 2023 Jan 4;15(1):179. doi: 10.3390/pharmaceutics15010179. Pharmaceutics. 2023. PMID: 36678808 Free PMC article.
References
-
- Leiter U., Keim U., Garbe C. Advances in Experimental Medicine and Biology. Volume 1268. Springer; Berlin, Germany: 2020. Epidemiology of skin cancer: Update; pp. 123–139. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

