Graves' Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review)
- PMID: 33920721
- PMCID: PMC8073133
- DOI: 10.3390/cancers13081944
Graves' Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review)
Abstract
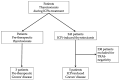
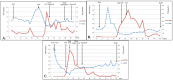
Thyrotoxicosis is an adverse event associated with immune checkpoint inhibitors (ICPis) that occurs in 0.6 to 3.2% of treated patients, depending on ICPi class. Presentation usually consists of a biphasic thyroiditis with transient thyrotoxicosis and secondary hypothyroidism. ICPi-induced Graves' disease (GD), due to the stimulating activity of TSH-receptor autoantibodies (TRAb), is extremely rare. The aim of this retrospective study was to describe the characteristics and evolution of GD during ICPi therapy. Five among 243 patients followed for ICPi-induced thyrotoxicosis showed TRAb positivity (2% of the cohort). GD occurred quickly after initiation of ICPis; its course was typical for two patients, with prolonged requirement for antithyroid drug treatment (ATD). The three other patients experienced biphasic thyroiditis with secondary hypothyroidism requiring long-term substitution. Three other patients had a diagnosis of GD before starting ICPis; they evolved toward hypothyroidism with early cessation of ATD and long-term substitution treatment during ICPi treatment. None developed significant Graves' orbitopathy. ICPi treatment was not interrupted for thyroid dysfunction. In conclusion, GD is a rare, immune-related adverse event of ICPis with an unusual course and frequent evolution to biphasic thyroiditis. In the case of ICPi-induced thyrotoxicosis in the presence of TRAb, observing the spontaneous evolution and performing a scintigraphy are useful before starting ATD treatment. Pre-existing GD is not exacerbated by ICPis and tends to evolve towards hypothyroidism. ICPi treatment can be maintained with adequate biochemical surveillance.
Keywords: Graves’ disease; endocrine toxicities; immune checkpoint inhibitors; immune-related adverse event; thyroid dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Usefulness of the 2nd generation assay for anti-TSH receptor antibodies to differentiate relapse of Graves' thyrotoxicosis from development of painless thyroiditis after antithyroid drug treatment for Graves' disease.Endocr J. 2005 Aug;52(4):493-7. doi: 10.1507/endocrj.52.493. Endocr J. 2005. PMID: 16127219 Clinical Trial.
-
ICPi-Induced Graves' Disease with Pre-existing Autoimmune Thyroid Disorders: A Case Report and Literature Review.Endocr Metab Immune Disord Drug Targets. 2025 Feb 10. doi: 10.2174/0118715303317264250116095028. Online ahead of print. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39950295
-
Graves' Disease Induced by Immune Checkpoint Inhibitors: A Case Report and Review of the Literature.Eur Thyroid J. 2019 Jul;8(4):192-195. doi: 10.1159/000501824. Epub 2019 Jul 9. Eur Thyroid J. 2019. PMID: 31602361 Free PMC article.
-
Measuring TSH receptor antibody to influence treatment choices in Graves' disease.Clin Endocrinol (Oxf). 2017 May;86(5):652-657. doi: 10.1111/cen.13327. Clin Endocrinol (Oxf). 2017. PMID: 28295509 Review.
-
Thyroid dysfunction following pregnancy and implications for breastfeeding.Best Pract Res Clin Endocrinol Metab. 2020 Jul;34(4):101438. doi: 10.1016/j.beem.2020.101438. Epub 2020 Jun 20. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 32651061 Review.
Cited by
-
Thyroid-related adverse events induced by immune checkpoint inhibitors.Front Endocrinol (Lausanne). 2022 Sep 20;13:1010279. doi: 10.3389/fendo.2022.1010279. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36204105 Free PMC article. Review.
-
Hypothyroidism After Use of Immune Checkpoint Inhibitor Therapy in Patient With Graves' Disease: Cure?JCEM Case Rep. 2022 Dec 3;1(1):luac024. doi: 10.1210/jcemcr/luac024. eCollection 2023 Jan. JCEM Case Rep. 2022. PMID: 37908246 Free PMC article.
-
A novel chromatin regulator-related immune checkpoint related gene prognostic signature and potential candidate drugs for endometrial cancer patients.Hereditas. 2022 Oct 18;159(1):40. doi: 10.1186/s41065-022-00253-w. Hereditas. 2022. PMID: 36253800 Free PMC article.
-
Immune checkpoint inhibitor-induced hypothyroidism predicts treatment response in Japanese subjects.Front Endocrinol (Lausanne). 2023 Jul 31;14:1221723. doi: 10.3389/fendo.2023.1221723. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37583431 Free PMC article.
-
Predictors of thyroid adverse events during cancer immunotherapy: a real-life experience at a single center.J Endocrinol Invest. 2023 Nov;46(11):2399-2409. doi: 10.1007/s40618-023-02096-2. Epub 2023 Apr 20. J Endocrinol Invest. 2023. PMID: 37076759
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

