Current Status of Putative Animal Sources of SARS-CoV-2 Infection in Humans: Wildlife, Domestic Animals and Pets
- PMID: 33920724
- PMCID: PMC8072559
- DOI: 10.3390/microorganisms9040868
Current Status of Putative Animal Sources of SARS-CoV-2 Infection in Humans: Wildlife, Domestic Animals and Pets
Abstract
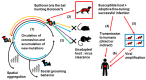
SARS-CoV-2 is currently considered to have emerged from a bat coronavirus reservoir. However, the real natural cycle of this virus remains to be elucidated. Moreover, the COVID-19 pandemic has led to novel opportunities for SARS-CoV-2 transmission between humans and susceptible animal species. In silico and in vitro evaluation of the interactions between the SARS-CoV-2 spike protein and eucaryotic angiotensin-converting enzyme 2 (ACE2) receptor have tentatively predicted susceptibility to SARS-CoV-2 infection of several animal species. Although useful, these data do not always correlate with in vivo data obtained in experimental models or during natural infections. Other host biological properties may intervene such as the body temperature, level of receptor expression, co-receptor, restriction factors, and genetic background. The spread of SARS-CoV-2 also depends on the extent and duration of viral shedding in the infected host as well as population density and behaviour (group living and grooming). Overall, current data indicate that the most at-risk interactions between humans and animals for COVID-19 infection are those involving certain mustelids (such as minks and ferrets), rodents (such as hamsters), lagomorphs (especially rabbits), and felines (including cats). Therefore, special attention should be paid to the risk of SARS-CoV-2 infection associated with pets.
Keywords: COVID-19; SARS-CoV-2; animal reservoirs; companion animals; domestic animals; modes of transmission; pets; wild animals; zoonosis.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

