Antitumor Activity of Pulvomycin via Targeting Activated-STAT3 Signaling in Docetaxel-Resistant Triple-Negative Breast Cancer Cells
- PMID: 33920736
- PMCID: PMC8074004
- DOI: 10.3390/biomedicines9040436
Antitumor Activity of Pulvomycin via Targeting Activated-STAT3 Signaling in Docetaxel-Resistant Triple-Negative Breast Cancer Cells
Abstract
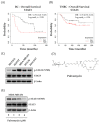
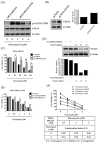
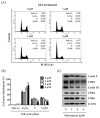
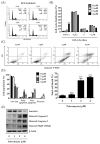
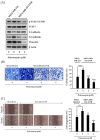
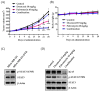
Although docetaxel-based regimens are common and effective for early-stage triple-negative breast cancer (TNBC) treatment, acquired drug resistance frequently occurs. Therefore, a novel therapeutic strategy for docetaxel-resistant TNBC is urgently required. Signal transducer and activator of transcription 3 (STAT3) plays a pivotal role in the tumorigenesis and metastasis of numerous cancers, and STAT3 signaling is aberrantly activated in TNBC cells. In this study, a docetaxel-resistant TNBC cell line (MDA-MB-231-DTR) was established, and mechanisms for the antitumor activity of pulvomycin, a novel STAT3 inhibitor isolated from marine-derived actinomycete, were investigated. Levels of activated STAT3 (p-STAT3 (Y705)) increased in docetaxel-resistant cells, and knockdown of STAT3 recovered the sensitivity to docetaxel in MDA-MB-231-DTR cells. Pulvomycin effectively inhibited the proliferation of both cell lines. In addition, pulvomycin suppressed the activation of STAT3 and subsequently induced G0/G1 cell cycle arrest and apoptosis. Pulvomycin also significantly inhibited the invasion and migration of MDA-MB-231-DTR cells through the modulation of epithelial-mesenchymal transition markers. In an MDA-MB-231-DTR-bearing xenograft mouse model, the combination of pulvomycin and docetaxel effectively inhibited tumor growth through STAT3 regulation. Thus, our findings demonstrate that the combination of docetaxel and STAT3 inhibitors is an effective strategy for overcoming docetaxel resistance in TNBC.
Keywords: docetaxel; metastasis; pulvomycin; resistance; signal transducer and activator of transcription 3; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Design, Synthesis, and Biological Activity of Marinacarboline Analogues as STAT3 Pathway Inhibitors for Docetaxel-Resistant Triple-Negative Breast Cancer.J Med Chem. 2023 Feb 23;66(4):3106-3133. doi: 10.1021/acs.jmedchem.2c01115. Epub 2023 Feb 14. J Med Chem. 2023. PMID: 36786551
-
Sequential combination of docetaxel with a SHP-1 agonist enhanced suppression of p-STAT3 signaling and apoptosis in triple negative breast cancer cells.J Mol Med (Berl). 2017 Sep;95(9):965-975. doi: 10.1007/s00109-017-1549-x. Epub 2017 Jun 4. J Mol Med (Berl). 2017. PMID: 28578456
-
Bufotalin Suppresses Proliferation and Metastasis of Triple-Negative Breast Cancer Cells by Promoting Apoptosis and Inhibiting the STAT3/EMT Axis.Molecules. 2023 Sep 23;28(19):6783. doi: 10.3390/molecules28196783. Molecules. 2023. PMID: 37836626 Free PMC article.
-
STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review.J Exp Clin Cancer Res. 2019 May 14;38(1):195. doi: 10.1186/s13046-019-1206-z. J Exp Clin Cancer Res. 2019. PMID: 31088482 Free PMC article.
-
Potential therapeutic targets of the JAK2/STAT3 signaling pathway in triple-negative breast cancer.Front Oncol. 2024 Apr 18;14:1381251. doi: 10.3389/fonc.2024.1381251. eCollection 2024. Front Oncol. 2024. PMID: 38699644 Free PMC article. Review.
Cited by
-
Enhanced anti-tumor efficacy of S3I-201 in breast cancer mouse model through Wharton jelly- exosome.Cancer Cell Int. 2024 Sep 18;24(1):318. doi: 10.1186/s12935-024-03501-3. Cancer Cell Int. 2024. PMID: 39294673 Free PMC article.
-
circUBR5 promotes ribosome biogenesis and induces docetaxel resistance in triple-negative breast cancer cell lines via the miR-340-5p/CMTM6/c-MYC axis.Neoplasia. 2025 Jan;59:101062. doi: 10.1016/j.neo.2024.101062. Epub 2024 Dec 12. Neoplasia. 2025. PMID: 39672097 Free PMC article.
-
Overcoming Acquired Drug Resistance to Cancer Therapies through Targeted STAT3 Inhibition.Int J Mol Sci. 2023 Mar 1;24(5):4722. doi: 10.3390/ijms24054722. Int J Mol Sci. 2023. PMID: 36902166 Free PMC article. Review.
-
Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies.J Hematol Oncol. 2022 Apr 12;15(1):44. doi: 10.1186/s13045-022-01260-0. J Hematol Oncol. 2022. PMID: 35414025 Free PMC article. Review.
-
Targeting STAT3 for Cancer Therapy: Focusing on Y705, S727, or Dual Inhibition?Cancers (Basel). 2025 Feb 23;17(5):755. doi: 10.3390/cancers17050755. Cancers (Basel). 2025. PMID: 40075607 Free PMC article. Review.
References
-
- Reddy S.M., Barcenas C.H., Sinha A.K., Hsu L., Moulder S.L., Tripathy D., Hortobagyi G.N., Valero V. Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br. J. Cancer. 2018;118:17–23. doi: 10.1038/bjc.2017.379. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

