Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Therapy with Direct-Acting Antivirals. A Systematic Review and Meta-Analysis
- PMID: 33920785
- PMCID: PMC8071154
- DOI: 10.3390/jcm10081694
Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Therapy with Direct-Acting Antivirals. A Systematic Review and Meta-Analysis
Abstract
Background: Hepatocellular carcinoma (HCC) is a major cause of morbidity and mortality among patients with cirrhosis. The risk of HCC recurrence after a complete response among patients treated with direct-acting antivirals (DAAs) has not been fully elucidated yet.
Aim: To assess the risk of HCC recurrence after DAA therapy for hepatitis C virus (HCV).
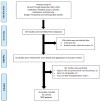
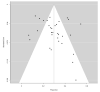
Methods: A systematic review across PubMed, Scopus and Scholar up to November 2020, including full-text studies that assessed the pattern of HCC recurrence after DAA therapy for HCV. Random-effect meta-analysis and univariable metaregression were applied to obtain pooled estimates for proportions and relative risk (RR) and variables influential for the outcome, respectively.
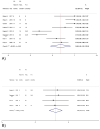
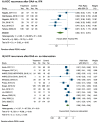
Results: Thirty-one studies with 2957 patients were included. Overall, 30% (CI, 26-34%) of the patients with a history of HCC experienced HCC recurrence after DAA therapy, at mean time intervals ranging from 4 to 21 months. This result increased when going from European studies (23%, CI, 17-28%) to US studies (34%, CI, 30-38%), to Egyptian studies (37%, CI, 27-47%), and to Asian studies (33%, CI, 27-40%). Sixty-eight percent (CI, 45-91%) of recurrent HCCs developed within 6 months of follow-up since DAA treatment, among the eight studies providing stratified data. Among the studies providing head-to-head comparisons, the HCC recurrence risk was significantly lower after DAA therapy than IFN (RR, 0.64; CI, 0.51-0.81), and after DAA therapy than no intervention (RR, 0.68; CI, 0.49-0.94).
Conclusions: The recurrence of HCC after DAA is not negligible, being higher soon after the end of treatment and among non-European countries. DAA therapy seems to reduce the risk of HCC recurrence compared to an IFN regimen and no intervention.
Keywords: direct-acting antivirals (DAAs); hepatitis C virus (HCV); hepatocellular carcinoma (HCC).
Conflict of interest statement
There are no conflicts of interest, either personal or financial, to declare by any of the authors.
Figures

References
-
- Reddy S.K., Steel J.L., Chen H.-W., DeMateo D.J., Cardinal J., Behari J., Humar A., Marsh J.W., Geller D.A., Tsung A. Outcomes of Curative Treatment for Hepatocellular Cancer in Nonalcoholic Steatohepatitis versus Hepatitis C and Alcoholic Liver Disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

