Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation
- PMID: 33920812
- PMCID: PMC8071131
- DOI: 10.3390/antibiotics10040439
Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation
Abstract
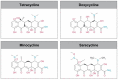
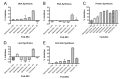
Prolonged broad-spectrum antibiotic use is more likely to induce bacterial resistance and dysbiosis of skin and gut microflora. First and second-generation tetracycline-class antibiotics have similar broad-spectrum antibacterial activity. Targeted tetracycline-class antibiotics are needed to limit antimicrobial resistance and improve patient outcomes. Sarecycline is a narrow-spectrum, third-generation tetracycline-class antibiotic Food and Drug Administration (FDA)-approved for treating moderate-to-severe acne. In vitro studies demonstrated activity against clinically relevant Gram-positive bacteria but reduced activity against Gram-negative bacteria. Recent studies have provided insight into how the structure of sarecycline, with a unique C7 moiety, interacts with bacterial ribosomes to block translation and prevent antibiotic resistance. Sarecycline reduces Staphylococcus aureus DNA and protein synthesis with limited effects on RNA, lipid, and bacterial wall synthesis. In agreement with in vitro data, sarecycline demonstrated narrower-spectrum in vivo activity in murine models of infection, exhibiting activity against S. aureus, but reduced efficacy against Escherichia coli compared to doxycycline and minocycline. In a murine neutropenic thigh wound infection model, sarecycline was as effective as doxycycline against S. aureus. The anti-inflammatory activity of sarecycline was comparable to doxycycline and minocycline in a rat paw edema model. Here, we review the antibacterial mechanisms of sarecycline and report results of in vivo studies of infection and inflammation.
Keywords: acne; animal models; antibiotic; antibiotic resistance; infection; inflammation; narrow-spectrum; sarecycline; tetracyclines.
Conflict of interest statement
C.G.B. reports research grants, consulting fees, speaking honoraria, and personal fees from Almirall. J.K. serves as a consultant for Almirall. S.K.T. is an employee of Paratek Pharma. G.D. reports research consulting fees and speaking honoraria from Almirall, Novartis, Amgen, and Galderma. J.L.J. is the owner and editor of DerMEDit and receives compensation from Almirall for medical writing assistance. A.G. is the Director of R&D and Medical Affairs at Almirall U.S.
Figures



Similar articles
-
Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris.Antimicrob Agents Chemother. 2018 Dec 21;63(1):e01297-18. doi: 10.1128/AAC.01297-18. Print 2019 Jan. Antimicrob Agents Chemother. 2018. PMID: 30397052 Free PMC article.
-
Sarecycline: A Review of Preclinical and Clinical Evidence.Clin Cosmet Investig Dermatol. 2020 Aug 13;13:553-560. doi: 10.2147/CCID.S190473. eCollection 2020. Clin Cosmet Investig Dermatol. 2020. PMID: 32884318 Free PMC article. Review.
-
Profiling the Effects of Systemic Antibiotics for Acne, Including the Narrow-Spectrum Antibiotic Sarecycline, on the Human Gut Microbiota.Front Microbiol. 2022 May 31;13:901911. doi: 10.3389/fmicb.2022.901911. eCollection 2022. Front Microbiol. 2022. PMID: 35711781 Free PMC article.
-
Sarecycline hydrochloride for the treatment of acne vulgaris.Drugs Today (Barc). 2019 Oct;55(10):615-625. doi: 10.1358/dot.2019.55.10.3045040. Drugs Today (Barc). 2019. PMID: 31720559 Review.
-
SARECYCLINE AND THE NARROW-SPECTRUM TETRACYCLINE CONCEPT: Currently Available Data and Potential Clinical Relevance in Dermatology.J Clin Aesthet Dermatol. 2020 Oct;13(10):45-48. Epub 2020 Oct 1. J Clin Aesthet Dermatol. 2020. PMID: 33584958 Free PMC article. Review.
Cited by
-
Sarecycline inhibits protein translation in Cutibacterium acnes 70S ribosome using a two-site mechanism.Nucleic Acids Res. 2023 Apr 11;51(6):2915-2930. doi: 10.1093/nar/gkad103. Nucleic Acids Res. 2023. PMID: 36864821 Free PMC article.
-
Efficacy and Safety of Sarecycline in Chinese Patients with Moderate-to-Severe Acne Vulgaris: Randomized Phase 3 Clinical Trial with Open-Label Follow-Up.Dermatol Ther (Heidelb). 2025 Aug 28. doi: 10.1007/s13555-025-01526-8. Online ahead of print. Dermatol Ther (Heidelb). 2025. PMID: 40877730
-
Combinations of Antibiotics Effective against Extensively- and Pandrug-Resistant Acinetobacter baumannii Patient Isolates.Microorganisms. 2024 Jul 2;12(7):1353. doi: 10.3390/microorganisms12071353. Microorganisms. 2024. PMID: 39065123 Free PMC article.
-
Effect of Sarecycline on the Acne Symptom and Impact Scale and Concerns in Moderate-to-Severe Truncal Acne in Open-Label Pilot Study.Antibiotics (Basel). 2023 Jan 5;12(1):94. doi: 10.3390/antibiotics12010094. Antibiotics (Basel). 2023. PMID: 36671294 Free PMC article.
-
A Retrospective Review of a Cohort of Patients with Periorificial Dermatitis Treated with Sarecycline.J Clin Aesthet Dermatol. 2024 Jun;17(6):50-54. J Clin Aesthet Dermatol. 2024. PMID: 38912196 Free PMC article.
References
-
- Del Rosso J.Q., Webster G.F., Rosen T., Thiboutot D., Leyden J.J., Gallo R., Walker C., Zhanel G., Eichenfield L. Status Report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1: Antibiotic Prescribing Patterns, Sources of Antibiotic Exposure, Antibiotic Consumption and Emergence of Antibiotic Resistance, Impact of Alterations in Antibiotic Prescribing, and Clinical Sequelae of Antibiotic Use. J. Clin. Aesthetic Dermatol. 2016;9:18–24. - PMC - PubMed
-
- Talkington K. The U.S. Is Not Prepared to Combat ‘Existential Threat’ of Antibiotic-Resistant Superbugs. PEW Charitable Trusts. [(accessed on 8 November 2020)]; Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2020/07/27/t....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

