Ursolic Acid Lactone Obtained from Eucalyptus tereticornis Increases Glucose Uptake and Reduces Inflammatory Activity and Intracellular Neutral Fat: An In Vitro Study
- PMID: 33920841
- PMCID: PMC8071196
- DOI: 10.3390/molecules26082282
Ursolic Acid Lactone Obtained from Eucalyptus tereticornis Increases Glucose Uptake and Reduces Inflammatory Activity and Intracellular Neutral Fat: An In Vitro Study
Abstract
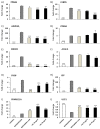
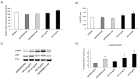
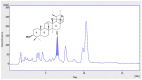
Obesity has a strong relationship to insulin resistance and diabetes mellitus, a chronic metabolic disease that alters many physiological functions. Naturally derived drugs have aroused great interest in treating obesity, and triterpenoids are natural compounds with multiple biological activities and antidiabetic mechanisms. Here, we evaluated the bioactivity of ursolic acid lactone (UAL), a lesser-known triterpenoid, obtained from Eucalyptus tereticornis. We used different cell lines to show for the first time that this molecule exhibits anti-inflammatory properties in a macrophage model, increases glucose uptake in insulin-resistant muscle cells, and reduces triglyceride content in hepatocytes and adipocytes. In 3T3-L1 adipocytes, UAL inhibited the expression of genes involved in adipogenesis and lipogenesis, enhanced the expression of genes involved in fat oxidation, and increased AMP-activated protein kinase phosphorylation. The range of biological activities demonstrated in vitro indicates that UAL is a promising molecule for fighting diabetes.
Keywords: adipocytes; diabetes; hepatocytes; macrophages; myocytes; ursolic acid lactone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
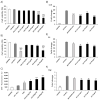
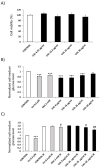
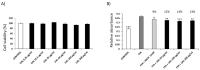

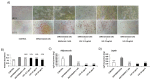
References
-
- International Diabetes Federation . IDF Diabetes Atla. 9th ed. Volume 2019 International Diabetes Federation; Brussels, Belgium: 2019.
-
- Schnurr T.M., Jakupović H., Carrasquilla G.D., Ängquist L., Grarup N., Sørensen T.I.A., Tjønneland A., Overvad K., Pedersen O., Hansen T., et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia. 2020 doi: 10.1007/s00125-020-05140-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

