Targeting the Sphingosine Kinase/Sphingosine-1-Phosphate Signaling Axis in Drug Discovery for Cancer Therapy
- PMID: 33920887
- PMCID: PMC8071327
- DOI: 10.3390/cancers13081898
Targeting the Sphingosine Kinase/Sphingosine-1-Phosphate Signaling Axis in Drug Discovery for Cancer Therapy
Abstract
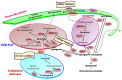
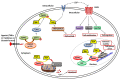
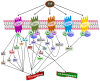
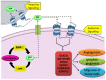
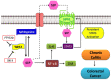
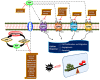
Sphingolipid metabolites have emerged as critical players in the regulation of various physiological processes. Ceramide and sphingosine induce cell growth arrest and apoptosis, whereas sphingosine-1-phosphate (S1P) promotes cell proliferation and survival. Here, we present an overview of sphingolipid metabolism and the compartmentalization of various sphingolipid metabolites. In addition, the sphingolipid rheostat, a fine metabolic balance between ceramide and S1P, is discussed. Sphingosine kinase (SphK) catalyzes the synthesis of S1P from sphingosine and modulates several cellular processes and is found to be essentially involved in various pathophysiological conditions. The regulation and biological functions of SphK isoforms are discussed. The functions of S1P, along with its receptors, are further highlighted. The up-regulation of SphK is observed in various cancer types and is also linked to radio- and chemoresistance and poor prognosis in cancer patients. Implications of the SphK/S1P signaling axis in human pathologies and its inhibition are discussed in detail. Overall, this review highlights current findings on the SphK/S1P signaling axis from multiple angles, including their functional role, mechanism of activation, involvement in various human malignancies, and inhibitor molecules that may be used in cancer therapy.
Keywords: cancer therapy; drug design and discovery; kinase inhibitors; sphingosine kinase; sphingosine metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Espaillat M.P., Shamseddine A.A., Adada M.M., Hannun Y.A., Obeid L.M. Ceramide and sphingosine-1-phosphate in cancer, two faces of the sphinx. Transl. Cancer Res. 2015;4:484–499.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

