Contemporary Neoadjuvant Therapies for High-Risk Melanoma: A Systematic Review
- PMID: 33920967
- PMCID: PMC8071293
- DOI: 10.3390/cancers13081905
Contemporary Neoadjuvant Therapies for High-Risk Melanoma: A Systematic Review
Abstract
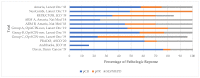
Despite advances in adjuvant immuno- and targeted therapies, the risk of relapse for stage III melanoma remains high. With 43 active entries on clinicaltrials.gov (8 July 2020), there is a surge of interest in the role of contemporary therapies in the neoadjuvant setting. We conducted a systematic review of trials performed in the last decade evaluating neoadjuvant targeted, immuno- or intralesional therapy for resectable stage III or IV melanoma. Database searches of Medline, Embase, and the Cochrane Central Register of Controlled Trials were conducted from inception to 13 February 2020. Two reviewers assessed titles, abstracts, and full texts. Trials investigating contemporary neoadjuvant therapies in high-risk melanoma were included. Eight phase II trials (4 randomized and 4 single-arm) involving 450 patients reported on neoadjuvant anti-BRAF/MEK targeted therapy (3), anti-PD-1/CTLA-4 immunotherapy (3), and intralesional therapy (2). The safest and most efficacious regimens were dabrafenib/trametinib and combination ipilimumab (1 mg/kg) + nivolumab (3 mg/kg). Pathologic complete response (pCR) and adverse events were comparable. Ipilimumab + nivolumab exhibited longer RFS. Contemporary neoadjuvant therapies are not only safe, but also demonstrate remarkable pCR and RFS-outcomes which are regarded as meaningful surrogates for long-term survival. Studies defining predictors of pCR, its correlation with oncologic outcomes, and phase III trials comparing neoadjuvant therapy to standard of care will be crucial.
Keywords: immunotherapy; intralesional therapy; melanoma; neoadjuvant; targeted therapy.
Conflict of interest statement
C.N. has received honoraria for speakerships from Merck, Novartis and EMD Sorono, she has also received honoraria for being on Advisory Boards for Novartis and Sanofi. M. Ong has been a consultant to and received honoraria from Bristol-Myers Squibb, Merck, AstraZeneca and Roche/Genentech. X.S. has been a consultant to and received honoraria from Bristol-Myers Squibb and Merck. All other authors, including the primary author K.B. and secondary author S.A., have no conflicts to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

