A Simplified SARS-CoV-2 Pseudovirus Neutralization Assay
- PMID: 33920999
- PMCID: PMC8071407
- DOI: 10.3390/vaccines9040389
A Simplified SARS-CoV-2 Pseudovirus Neutralization Assay
Abstract
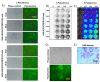
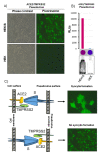
COVID-19 is an ongoing pandemic caused by the highly infectious coronavirus SARS-CoV-2 that is engaging worldwide scientific research to find a timely and effective eradication strategy. Great efforts have been put into anti-COVID-19 vaccine generation in an effort to protect the world population and block SARS-CoV-2 spread. To validate the protective efficacy of the vaccination campaign and effectively control the pandemic, it is necessary to quantify the induction of neutralizing antibodies by vaccination, as they have been established to be a correlate of protection. In this work, a SARS-CoV-2 pseudovirus neutralization assay, based on a replication-incompetent lentivirus expressing an adapted form of CoV-2 S protein and an ACE2/TMPRSS2 stably expressing cell line, has been minimized in terms of protocol steps without loss of accuracy. The goal of the present simplified neutralization system is to improve SARS-CoV-2 vaccination campaign by means of an easy and accessible approach to be performed in any medical laboratory, maintaining the sensitivity and quantitative reliability of classical serum neutralization assays. Further, this assay can be easily adapted to different coronavirus variants by simply modifying the pseudotyping vector.
Keywords: COVID-19; SARS-CoV-2; neutralization assay; neutralizing antibody; pseudovirus.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

