Rapamycin Alleviates 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Aggravated Dermatitis in Mice with Imiquimod-Induced Psoriasis-Like Dermatitis by Inducing Autophagy
- PMID: 33921372
- PMCID: PMC8069848
- DOI: 10.3390/ijms22083968
Rapamycin Alleviates 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Aggravated Dermatitis in Mice with Imiquimod-Induced Psoriasis-Like Dermatitis by Inducing Autophagy
Abstract
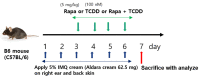
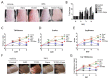
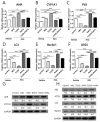
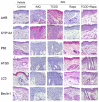
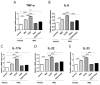
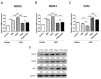
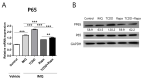
Recently, the mTOR signaling has emerged as an important player in the pathogenesis of psoriasis. We previously found that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced psoriatic skin inflammation was related to the inhibition of autophagy in keratinocytes. However, the effects and detailed molecular mechanisms of the mTOR inhibitor rapamycin and TCDD on psoriasis in vivo remain to be elucidated. In this study, we aimed to evaluate the effects of rapamycin and TCDD on skin lesions in imiquimod (IMQ)-induced psoriasis using a mouse model. TCDD aggravated skin inflammation in an IMQ-induced psoriatic mouse model. Furthermore, TCDD increased the expression of aryl hydrocarbon receptor (AHR), CYP1A1, proinflammatory cytokines, oxidative stress markers (NADPH oxidase (Nox) 2, Nox4), and phosphorylated P65NF-ĸB, whereas the expression of autophagy-related factors and the antioxidant marker nuclear factor-erythroid 2-related factor 2 (NRF2) decreased. Rapamycin reduced the aggravated skin inflammation induced by TCDD and restored TCDD-induced autophagy suppression and the increase of AHR expression, oxidative stress, and inflammatory response in the skin lesions of a psoriatic mouse model. In conclusion, we demonstrated that rapamycin alleviates TCDD-induced aggravated dermatitis in mice with imiquimod-induced psoriasis-like dermatitis through AHR and autophagy modulation.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor; autophagy; psoriasis; rapamycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Santus P., Rizzi M., Radovanovic D., Airoldi A., Cristiano A., Conic R., Petrou S., Pigatto P.D.M., Bragazzi N., Colombo D., et al. Psoriasis and Respiratory Comorbidities: The Added Value of Fraction of Exhaled Nitric Oxide as a New Method to Detect, Evaluate, and Monitor Psoriatic Systemic Involvement and Therapeutic Efficacy. BioMed Res. Int. 2018;2018:3140682. doi: 10.1155/2018/3140682. - DOI - PMC - PubMed
-
- Conic R.R., Damiani G., Schrom K.P., Ramser A.E., Zheng C., Xu R., McCormick T.S., Cooper K.D. Psoriasis and Psoriatic Arthritis Cardiovascular Disease Endotypes Identified by Red Blood Cell Distribution Width and Mean Platelet Volume. J. Clin. Med. 2020;9:186. doi: 10.3390/jcm9010186. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

