Synthesis of 4-Arylselanyl-1 H-1,2,3-triazoles from Selenium-Containing Carbinols
- PMID: 33921473
- PMCID: PMC8070154
- DOI: 10.3390/molecules26082224
Synthesis of 4-Arylselanyl-1 H-1,2,3-triazoles from Selenium-Containing Carbinols
Abstract
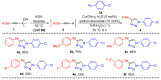
In this work, we present a simple way to achieve 4-arylselanyl-1H-1,2,3-triazoles from selenium-containing carbinols in a one-pot strategy. The selenium-containing carbinols were used as starting materials to produce a range of selanyl-triazoles in moderate to good yields, including a quinoline and Zidovudine derivatives. One-pot protocols are crucial to the current concerns about waste production and solvent consumption, avoiding the isolation and purification steps of the reactive terminal selanylalkynes. We could also isolate an interesting and unprecedented by-product with one alkynylselenium moiety connected to the triazole.
Keywords: 1,2,3-triazoles; carbinols; click chemistry; cycloaddition; heterocycles; selenium.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

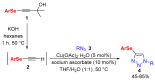

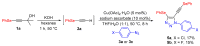
References
-
- Dehaen W., Bakulev V.A., editors. Chemistry of 1,2,3-Triazoles. Volume 40. Springer International Publishing; Cham, Switzerland: 2015. Topics in Heterocyclic Chemistry.
-
- Themed collection: Click chemistry: Function follows form. Chem. Soc. Rev. 2010;39:1221–1407. - PubMed
-
- Costa G.P., Baldinotti R.S.M., Fronza M.G., Nascimento J.E.R., Dias I.F.C., Sonego M.S., Seixas F.K., Collares T., Perin G., Jacob R.G., et al. Synthesis, Molecular Docking, and Preliminary Evaluation of 2-(1,2,3-Triazoyl)benzaldehydes as Multifunctional Agents for the Treatment of Alzheimer’s Disease. ChemMedChem. 2020;15:610–622. doi: 10.1002/cmdc.201900622. - DOI - PubMed
-
- Begnini K.R., Duarte W.R., da Silva L.P., Buss J.H., Goldani B.S., Fronza M., Segatto N.V., Alves D., Savegnago L., Seixas F.K., et al. Apoptosis Induction by 7-Chloroquinoline-1,2,3-Triazoyl Carboxamides in Triple Negative Breast Cancer Cells. Biomed. Pharmacother. 2017;91:510–516. doi: 10.1016/j.biopha.2017.04.098. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

