Central Alteration in Peripheral Neuropathy of Trembler-J Mice: Hippocampal pmp22 Expression and Behavioral Profile in Anxiety Tests
- PMID: 33921657
- PMCID: PMC8074002
- DOI: 10.3390/biom11040601
Central Alteration in Peripheral Neuropathy of Trembler-J Mice: Hippocampal pmp22 Expression and Behavioral Profile in Anxiety Tests
Abstract
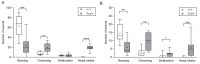
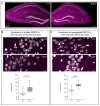
Charcot-Marie-Tooth (CMT) type 1 disease is the most common human hereditary demyelinating neuropathy. Mutations in pmp22 cause about 70% of all CMT1. Trembler-J (TrJ/+) mice are an animal model of CMT1E, having the same spontaneous pmp22 mutation that is found in humans. We compared the behavior profile of TrJ/+ and +/+ (wild-type) in open-field and elevated-plus-maze anxiety tests. In these tests, TrJ/+ showed an exclusive head shake movement, a lower frequency of rearing, but a greater frequency of grooming. In elevated-plus-maze, TrJ/+ defecate more frequently, performed fewer total entries, and have fewer entries to closed arms. These hippocampus-associated behaviors in TrJ/+ are consistent with increased anxiety levels. The expression of pmp22 and soluble PMP22 were evaluated in E17-hippocampal neurons and adult hippocampus by in situ hybridization and successive immunohistochemistry. Likewise, the expression of pmp22 was confirmed by RT-qPCR in the entire isolated hippocampi of both genotypes. Moreover, the presence of aggregated PMP22 was evidenced in unmasked granular hippocampal adult neurons and shows genotypic differences. We showed for the first time a behavior profile trait associated with anxiety and a differential expression of pmp22/PMP22 in hippocampal neurons of TrJ/+ and +/+ mice, demonstrating the involvement at the central level in an animal model of peripheral neuropathy (CMT1E).
Keywords: CA3 neurons; Charcot–Marie–Tooth; Trembler-J; anxiety; hippocampus; peripheral-myelin-protein-22.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice.Biology (Basel). 2023 Oct 10;12(10):1324. doi: 10.3390/biology12101324. Biology (Basel). 2023. PMID: 37887034 Free PMC article.
-
Colocalization Analysis of Peripheral Myelin Protein-22 and Lamin-B1 in the Schwann Cell Nuclei of Wt and TrJ Mice.Biomolecules. 2022 Mar 16;12(3):456. doi: 10.3390/biom12030456. Biomolecules. 2022. PMID: 35327648 Free PMC article.
-
Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22.J Neurosci. 1998 Jan 15;18(2):731-40. doi: 10.1523/JNEUROSCI.18-02-00731.1998. J Neurosci. 1998. PMID: 9425015 Free PMC article.
-
Comparison of a new pmp22 transgenic mouse line with other mouse models and human patients with CMT1A.J Anat. 2002 Apr;200(4):377-90. doi: 10.1046/j.1469-7580.2002.00039.x. J Anat. 2002. PMID: 12090404 Free PMC article. Review.
-
Rodent models with expression of PMP22: Relevance to dysmyelinating CMT and HNPP.J Neurol Sci. 2019 Mar 15;398:79-90. doi: 10.1016/j.jns.2019.01.030. Epub 2019 Jan 21. J Neurol Sci. 2019. PMID: 30685714 Review.
Cited by
-
Functional ultrasound and brain connectivity reveal central nervous system compromise in Trembler-J mice model of Charcot-Marie-Tooth disease.Sci Rep. 2024 Dec 3;14(1):30073. doi: 10.1038/s41598-024-80022-z. Sci Rep. 2024. PMID: 39627364 Free PMC article.
-
Animal Models as a Tool to Design Therapeutical Strategies for CMT-like Hereditary Neuropathies.Brain Sci. 2021 Sep 18;11(9):1237. doi: 10.3390/brainsci11091237. Brain Sci. 2021. PMID: 34573256 Free PMC article. Review.
-
In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice.Biology (Basel). 2023 Oct 10;12(10):1324. doi: 10.3390/biology12101324. Biology (Basel). 2023. PMID: 37887034 Free PMC article.
-
The Ketogenic Diet but not Hydroxycitric Acid Keeps Brain Mitochondria Quality Control and mtDNA Integrity Under Focal Stroke.Mol Neurobiol. 2023 Aug;60(8):4288-4303. doi: 10.1007/s12035-023-03325-8. Epub 2023 Apr 19. Mol Neurobiol. 2023. PMID: 37074549
-
Curcumin and Ethanol Effects in Trembler-J Schwann Cell Culture.Biomolecules. 2022 Mar 29;12(4):515. doi: 10.3390/biom12040515. Biomolecules. 2022. PMID: 35454103 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

