Genistein Activates Transcription Factor EB and Corrects Niemann-Pick C Phenotype
- PMID: 33921734
- PMCID: PMC8073251
- DOI: 10.3390/ijms22084220
Genistein Activates Transcription Factor EB and Corrects Niemann-Pick C Phenotype
Abstract
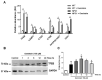
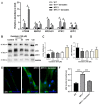
Niemann-Pick type C disease (NPCD) is a lysosomal storage disease (LSD) characterized by abnormal cholesterol accumulation in lysosomes, impaired autophagy flux, and lysosomal dysfunction. The activation of transcription factor EB (TFEB), a master lysosomal function regulator, reduces the accumulation of lysosomal substrates in LSDs where the degradative capacity of the cells is compromised. Genistein can pass the blood-brain barrier and activate TFEB. Hence, we investigated the effect of TFEB activation by genistein toward correcting the NPC phenotype. We show that genistein promotes TFEB translocation to the nucleus in HeLa TFEB-GFP, Huh7, and SHSY-5Y cells treated with U18666A and NPC1 patient fibroblasts. Genistein treatment improved lysosomal protein expression and autophagic flux, decreasing p62 levels and increasing those of the LC3-II in NPC1 patient fibroblasts. Genistein induced an increase in β-hexosaminidase activity in the culture media of NPC1 patient fibroblasts, suggesting an increase in lysosomal exocytosis, which correlated with a decrease in cholesterol accumulation after filipin staining, including cells treated with U18666A and NPC1 patient fibroblasts. These results support that genistein-mediated TFEB activation corrects pathological phenotypes in NPC models and substantiates the need for further studies on this isoflavonoid as a potential therapeutic agent to treat NPCD and other LSDs with neurological compromise.
Keywords: Niemann–Pick C; TFEB; cholesterol; genistein; lysosomal storage diseases; lysosome clearance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

