Systematic Review and Network Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for Treatment-Naïve ALK-Positive Lung Cancer
- PMID: 33921762
- PMCID: PMC8072703
- DOI: 10.3390/cancers13081966
Systematic Review and Network Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for Treatment-Naïve ALK-Positive Lung Cancer
Abstract
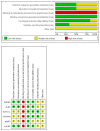
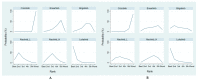
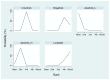
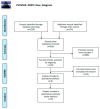
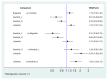
Several anaplastic lymphoma kinase inhibitors (ALKIs) have demonstrated excellent efficacy on overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and also better adverse effect (AE) profiles compared to cytotoxic chemotherapy in advanced stage anaplastic lymphoma kinase (ALK) rearrangement-positive non-small cell lung cancer (NSCLC) in phase III randomized clinical trials (RCTs). We conducted this systematic review and network meta-analysis to provide a ranking of ALKIs for treatment-naïve ALK-positive patients in terms of PFS, ORR, and AEs. In addition, a sub-group analysis of treatment benefits in patients with baseline brain metastasis was also conducted. Contrast-based analysis was performed for multiple treatment comparisons with the restricted maximum likelihood approach. Treatment rank was estimated using the surface under the cumulative ranking curve (SUCRA), as well as the probability of being the best (Prbest) reference. All next-generation ALKIs were superior to crizotinib in PFS but lorlatinib and brigatinib had increased AEs. The probability of lorlatinib being ranked first among all treatment arms was highest (SUCRA = 93.3%, Prbest = 71.8%), although there were no significant differences in pairwise comparisons with high- (600 mg twice daily) and low- (300 mg twice daily) dose alectinib. In subgroup analysis of patients with baseline brain metastasis, low-dose alectinib had the best PFS (SUCRA = 87.3%, Prbest = 74.9%). Lorlatinib was associated with the best ranking for ORR (SUCRA = 90.3%, Prbest = 71.3%), although there were no significant differences in pairwise comparisons with the other ALKIs. In addition, low-dose alectinib had the best safety performance (SUCRA = 99.4%, Prbest = 97.9%). Lorlatinib and low-dose alectinib had the best PFS and ORR in the overall population and baseline brain metastasis subgroup, respectively. Low-dose alectinib had the lowest AE risk among the available ALKIs. Further head-to-head large-scale phase III RCTs are needed to verify our conclusions.
Keywords: ALK inhibitor; alectinib; brigatinib; ceritinib; crizotinib; ensartinib; lorlatinib; network meta-analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Govindan R., Page N., Morgensztern D., Read W., Tierney R., Vlahiotis A., Spitznagel E.L., Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

